Toll-like receptor 7-mediated type I interferon signaling prevents cholestasis- and hepatotoxin-induced liver fibrosis
- PMID: 24375615
- PMCID: PMC4273749
- DOI: 10.1002/hep.26981
Toll-like receptor 7-mediated type I interferon signaling prevents cholestasis- and hepatotoxin-induced liver fibrosis
Abstract
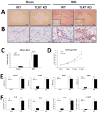
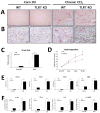
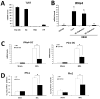
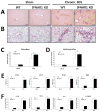
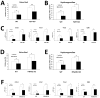
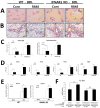
Toll-like receptor 7 (TLR7) signaling predominantly regulates production of type I interferons (IFNs), which has been suggested in clinical studies to be antifibrotic. However, the mechanistic role of the TLR7-type I IFN axis in liver fibrosis has not been elucidated. In the present study, liver fibrosis was induced in wild-type (WT), TLR7-deficient, and IFN-α/β receptor-1 (IFNAR1)-deficient mice and TLR7-mediated signaling was assessed in liver cells isolated from these mice. TLR7-deficient and IFNAR1-deficient mice were more susceptible to liver fibrosis than WT mice, indicating that TLR7-type I IFN signaling exerts a protective effect against liver fibrosis. Notably, the hepatic expression of interleukin-1 receptor antagonist (IL-1ra) was suppressed in TLR7- or IFNAR1-deficient mice compared with respective WT mice, and treatment with recombinant IL-1ra reduced liver fibrosis. In vivo activation of TLR7 significantly increased IFNa4 and IL-1ra expression in the liver. Interestingly, each cytokine had a different cellular source, showing that dendritic cells (DCs) are the responsible cell type for production of type I IFN, while Kupffer cells (KCs) mainly produce IL-1ra in response to type I IFN. Furthermore, TLR7 activation by R848 injection suppressed liver fibrosis and production of proinflammatory cytokines, and these effects were dependent on type I IFN signaling. Consistent with in vivo data, IFN-α significantly induced IL-1ra production in primary KCs.
Conclusion: TLR7 signaling activates DCs to produce type I IFN, which in turn induces antifibrogenic IL-1ra production in KCs. Thus, manipulation of the TLR7-type I IFN-IL-1ra axis may be a new therapeutic strategy for the treatment of liver fibrosis.
© 2014 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures

Similar articles
-
Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice.Gastroenterology. 2011 Feb;140(2):697-708.e4. doi: 10.1053/j.gastro.2010.08.020. Epub 2010 Aug 19. Gastroenterology. 2011. PMID: 20727895 Free PMC article.
-
Toll-Like Receptor-7 Signaling Promotes Nonalcoholic Steatohepatitis by Inhibiting Regulatory T Cells in Mice.Am J Pathol. 2018 Nov;188(11):2574-2588. doi: 10.1016/j.ajpath.2018.07.011. Epub 2018 Aug 18. Am J Pathol. 2018. PMID: 30125542
-
Toll-Like Receptor 5 Signaling Ameliorates Liver Fibrosis by Inducing Interferon β-Modulated IL-1 Receptor Antagonist in Mice.Am J Pathol. 2020 Mar;190(3):614-629. doi: 10.1016/j.ajpath.2019.11.012. Epub 2020 Jan 21. Am J Pathol. 2020. PMID: 31972159
-
Sex Differences in Primary HIV Infection: Revisiting the Role of TLR7-Driven Type 1 IFN Production by Plasmacytoid Dendritic Cells in Women.Front Immunol. 2021 Aug 27;12:729233. doi: 10.3389/fimmu.2021.729233. eCollection 2021. Front Immunol. 2021. PMID: 34512664 Free PMC article. Review.
-
The molecular basis for differential type I interferon signaling.J Biol Chem. 2017 May 5;292(18):7285-7294. doi: 10.1074/jbc.R116.774562. Epub 2017 Mar 13. J Biol Chem. 2017. PMID: 28289098 Free PMC article. Review.
Cited by
-
The role of 2'-5'-oligoadenylate synthase-like protein (OASL1) in biliary and hepatotoxin-induced liver injury in mice.Sci Rep. 2024 Sep 19;14(1):21873. doi: 10.1038/s41598-024-72465-1. Sci Rep. 2024. PMID: 39300174 Free PMC article.
-
Therapeutic Opportunities of IL-22 in Non-Alcoholic Fatty Liver Disease: From Molecular Mechanisms to Clinical Applications.Biomedicines. 2021 Dec 14;9(12):1912. doi: 10.3390/biomedicines9121912. Biomedicines. 2021. PMID: 34944732 Free PMC article. Review.
-
Role of Microbiota-Derived Metabolites in Alcoholic and Non-Alcoholic Fatty Liver Diseases.Int J Mol Sci. 2021 Dec 31;23(1):426. doi: 10.3390/ijms23010426. Int J Mol Sci. 2021. PMID: 35008852 Free PMC article. Review.
-
HRS plays an important role for TLR7 signaling to orchestrate inflammation and innate immunity upon EV71 infection.PLoS Pathog. 2017 Aug 30;13(8):e1006585. doi: 10.1371/journal.ppat.1006585. eCollection 2017 Aug. PLoS Pathog. 2017. PMID: 28854257 Free PMC article.
-
Mouse Type-I Interferon-Mannosylated Albumin Fusion Protein for the Treatment of Chronic Hepatitis.Pharmaceuticals (Basel). 2024 Feb 19;17(2):260. doi: 10.3390/ph17020260. Pharmaceuticals (Basel). 2024. PMID: 38399475 Free PMC article.
References
-
- Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 (Suppl 1):S38–53. - PubMed
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Eberle F, Sirin M, Binder M, Dalpke AH. Bacterial RNA is recognized by different sets of immunoreceptors. Eur J Immunol. 2009;39:2537–2547. - PubMed
-
- Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, et al. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol. 2009;10:587–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
