Psychological processing in chronic pain: a neural systems approach
- PMID: 24374383
- PMCID: PMC3944001
- DOI: 10.1016/j.neubiorev.2013.12.006
Psychological processing in chronic pain: a neural systems approach
Abstract
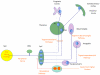
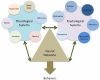
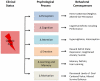
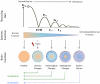
Our understanding of chronic pain involves complex brain circuits that include sensory, emotional, cognitive and interoceptive processing. The feed-forward interactions between physical (e.g., trauma) and emotional pain and the consequences of altered psychological status on the expression of pain have made the evaluation and treatment of chronic pain a challenge in the clinic. By understanding the neural circuits involved in psychological processes, a mechanistic approach to the implementation of psychology-based treatments may be better understood. In this review we evaluate some of the principle processes that may be altered as a consequence of chronic pain in the context of localized and integrated neural networks. These changes are ongoing, vary in their magnitude, and their hierarchical manifestations, and may be temporally and sequentially altered by treatments, and all contribute to an overall pain phenotype. Furthermore, we link altered psychological processes to specific evidence-based treatments to put forth a model of pain neuroscience psychology.
Keywords: Allostatic load; Amygdala; Anhedonia; Anterior cingulate cortex; Anxiety; Attention; Behavior; Brain; Catastrophizing; Chronic pain; Cognition; Depression; Fear; Hippocampus; Imaging; Insula; Interoception; Motivation; Neurocircuit; Nociception; Parietal cortex; Perception; Prefrontal cortex; Psychology; Reward.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
A Functional Magnetic Resonance Imaging Study to Investigate the Utility of a Picture Imagination Task in Investigating Neural Responses in Patients with Chronic Musculoskeletal Pain to Daily Physical Activity Photographs.PLoS One. 2015 Oct 23;10(10):e0141133. doi: 10.1371/journal.pone.0141133. eCollection 2015. PLoS One. 2015. PMID: 26496709 Free PMC article.
-
Psychological Processes in Chronic Pain: Influences of Reward and Fear Learning as Key Mechanisms - Behavioral Evidence, Neural Circuits, and Maladaptive Changes.Neuroscience. 2018 Sep 1;387:72-84. doi: 10.1016/j.neuroscience.2017.08.051. Epub 2017 Sep 7. Neuroscience. 2018. PMID: 28890049 Review.
-
Positive emotions and brain reward circuits in chronic pain.J Comp Neurol. 2016 Jun 1;524(8):1646-52. doi: 10.1002/cne.23968. Epub 2016 Feb 3. J Comp Neurol. 2016. PMID: 26788716 Free PMC article. Review.
-
Brain changes associated with cognitive and emotional factors in chronic pain: A systematic review.Eur J Pain. 2017 May;21(5):769-786. doi: 10.1002/ejp.1003. Epub 2017 Feb 1. Eur J Pain. 2017. PMID: 28146315 Review.
-
Anhedonia Following Early-Life Adversity Involves Aberrant Interaction of Reward and Anxiety Circuits and Is Reversed by Partial Silencing of Amygdala Corticotropin-Releasing Hormone Gene.Biol Psychiatry. 2018 Jan 15;83(2):137-147. doi: 10.1016/j.biopsych.2017.08.023. Epub 2017 Sep 7. Biol Psychiatry. 2018. PMID: 29033027 Free PMC article.
Cited by
-
Psychological Interventions for the Management of Chronic Pain: a Review of Current Evidence.Curr Pain Headache Rep. 2015 Sep;19(9):43. doi: 10.1007/s11916-015-0517-9. Curr Pain Headache Rep. 2015. PMID: 26209170 Review.
-
Self - Reported Depression, Anxiety and Evaluation of Own Pain in Clinical Sample of Patients with Different Location of Chronic Pain.Zdr Varst. 2014 Dec 30;54(1):1-10. doi: 10.1515/sjph-2015-0001. eCollection 2015 Mar. Zdr Varst. 2014. PMID: 27646616 Free PMC article.
-
Group II metabotropic glutamate receptor expressing neurons in anterior cingulate cortex become sensitized after inflammatory and neuropathic pain.Mol Pain. 2020 Jan-Dec;16:1744806920915339. doi: 10.1177/1744806920915339. Mol Pain. 2020. PMID: 32326814 Free PMC article.
-
Yoga and Low Back Pain: No Fool's Tool.Ann Intern Med. 2017 Jul 18;167(2):129-130. doi: 10.7326/M17-1263. Epub 2017 Jun 20. Ann Intern Med. 2017. PMID: 28631002 Free PMC article. No abstract available.
-
Provoked vestibulodynia: current perspectives.Int J Womens Health. 2017 Sep 11;9:631-642. doi: 10.2147/IJWH.S113416. eCollection 2017. Int J Womens Health. 2017. PMID: 28979166 Free PMC article. Review.
References
-
- Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Huang W, Burgess G, Machin I, Rice AS. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. European journal of pain. 2011 - PubMed
-
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. - PubMed
-
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004a;108:129–136. - PubMed
-
- Bailey KM, Carleton RN, Vlaeyen JW, Asmundson GJ. Treatments addressing pain-related fear and anxiety in patients with chronic musculoskeletal pain: a preliminary review. Cogn Behav Ther. 2010;39:46–63. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

