Cholinergic efferent synaptic transmission regulates the maturation of auditory hair cell ribbon synapses
- PMID: 24350389
- PMCID: PMC3843824
- DOI: 10.1098/rsob.130163
Cholinergic efferent synaptic transmission regulates the maturation of auditory hair cell ribbon synapses
Abstract
Spontaneous electrical activity generated by developing sensory cells and neurons is crucial for the maturation of neural circuits. The full maturation of mammalian auditory inner hair cells (IHCs) depends on patterns of spontaneous action potentials during a 'critical period' of development. The intrinsic spiking activity of IHCs can be modulated by inhibitory input from cholinergic efferent fibres descending from the brainstem, which transiently innervate immature IHCs. However, it remains unknown whether this transient efferent input to developing IHCs is required for their functional maturation. We used a mouse model that lacks the α9-nicotinic acetylcholine receptor subunit (α9nAChR) in IHCs and another lacking synaptotagmin-2 in the efferent terminals to remove or reduce efferent input to IHCs, respectively. We found that the efferent system is required for the developmental linearization of the Ca(2+)-sensitivity of vesicle fusion at IHC ribbon synapses, without affecting their general cell development. This provides the first direct evidence that the efferent system, by modulating IHC electrical activity, is required for the maturation of the IHC synaptic machinery. The central control of sensory cell development is unique among sensory systems.
Figures
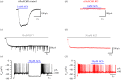
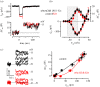
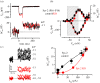
 , where c is a scaling coefficient and the power is N. The ΔCm traces shown on the left are averaged from 11 IHCs for both control and α9nAChR KO mice.
, where c is a scaling coefficient and the power is N. The ΔCm traces shown on the left are averaged from 11 IHCs for both control and α9nAChR KO mice.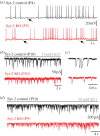
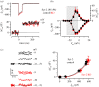
 .
.
 .
.Similar articles
-
Efferent Inhibition of the Cochlea.Cold Spring Harb Perspect Med. 2019 May 1;9(5):a033530. doi: 10.1101/cshperspect.a033530. Cold Spring Harb Perspect Med. 2019. PMID: 30082454 Free PMC article. Review.
-
mGluR1 enhances efferent inhibition of inner hair cells in the developing rat cochlea.J Physiol. 2017 Jun 1;595(11):3483-3495. doi: 10.1113/JP272604. Epub 2017 Apr 21. J Physiol. 2017. PMID: 28211069 Free PMC article.
-
Control of exocytosis by synaptotagmins and otoferlin in auditory hair cells.J Neurosci. 2010 Oct 6;30(40):13281-90. doi: 10.1523/JNEUROSCI.2528-10.2010. J Neurosci. 2010. PMID: 20926654 Free PMC article.
-
Developmental regulation of nicotinic synapses on cochlear inner hair cells.J Neurosci. 2004 Sep 8;24(36):7814-20. doi: 10.1523/JNEUROSCI.2102-04.2004. J Neurosci. 2004. PMID: 15356192 Free PMC article.
-
The auditory hair cell ribbon synapse: from assembly to function.Annu Rev Neurosci. 2012;35:509-28. doi: 10.1146/annurev-neuro-061010-113705. Annu Rev Neurosci. 2012. PMID: 22715884 Review.
Cited by
-
Specific synaptopathies diversify brain responses and hearing disorders: you lose the gain from early life.Cell Tissue Res. 2015 Jul;361(1):77-93. doi: 10.1007/s00441-015-2168-x. Epub 2015 Apr 7. Cell Tissue Res. 2015. PMID: 25843689 Free PMC article. Review.
-
Cochlea-Specific Deletion of Cav1.3 Calcium Channels Arrests Inner Hair Cell Differentiation and Unravels Pitfalls of Conditional Mouse Models.Front Cell Neurosci. 2019 May 22;13:225. doi: 10.3389/fncel.2019.00225. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31178698 Free PMC article.
-
The mammalian efferent vestibular system plays a crucial role in the high-frequency response and short-term adaptation of the vestibuloocular reflex.J Neurophysiol. 2015 Dec;114(6):3154-65. doi: 10.1152/jn.00307.2015. Epub 2015 Sep 30. J Neurophysiol. 2015. PMID: 26424577 Free PMC article.
-
Efferent Inhibition of the Cochlea.Cold Spring Harb Perspect Med. 2019 May 1;9(5):a033530. doi: 10.1101/cshperspect.a033530. Cold Spring Harb Perspect Med. 2019. PMID: 30082454 Free PMC article. Review.
-
Virally-Mediated Enhancement of Efferent Inhibition Reduces Acoustic Trauma in Wild Type Murine Cochleas.bioRxiv [Preprint]. 2024 Sep 15:2024.09.12.612688. doi: 10.1101/2024.09.12.612688. bioRxiv. 2024. PMID: 39314296 Free PMC article. Preprint.
References
-
- Fuchs PA. 2005. Time and intensity coding at the hair cell's ribbon synapse. J. Physiol. 566, 7–12 (doi:10.1113/jphysiol.2004.082214) - DOI - PMC - PubMed
-
- Marcotti W. 2012. Functional assembly of mammalian cochlear hair cells. Exp. Physiol. 97, 438–451 (doi:10.1113/expphysiol.2011.059303) - DOI - PMC - PubMed
-
- Johnson SL, Marcotti W, Kros CJ. 2005. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J. Physiol. 563, 177–191 (doi:10.1113/jphysiol.2004.074740) - DOI - PMC - PubMed
-
- Meyer AC, et al. 2009. Tuning of synapse number, structure and function in the cochlea. Nat. Neurosci. 12, 444–453 (doi:10.1038/nn.2293) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

