A RabGAP regulates life-cycle duration via trimeric G-protein cascades in Dictyostelium discoideum
- PMID: 24349132
- PMCID: PMC3859538
- DOI: 10.1371/journal.pone.0081811
A RabGAP regulates life-cycle duration via trimeric G-protein cascades in Dictyostelium discoideum
Abstract
Background: The life-cycle of cellular slime molds comprises chronobiologically regulated processes. During the growth phase, the amoeboid cells proliferate at a definite rate. Upon starvation, they synthesize cAMP as both first and second messengers in signalling pathways and form aggregates, migrating slugs, and fruiting bodies, consisting of spores and stalk cells, within 24 h. In Dictyostelium discoideum, because most growth-specific events cease during development, proliferative and heterochronic mutations are not considered to be interrelated and no genetic factor governing the entire life-cycle duration has ever been identified.
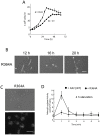
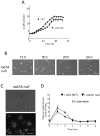
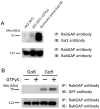
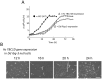
Methodology/principal findings: Using yeast 2-hybrid library screening, we isolated a Dictyostelium discoideum RabGAP, Dd Rbg-3, as a candidate molecule by which the Dictyostelium Gα2 subunit directs its effects. Rab GTPase-activating protein, RabGAP, acts as a negative regulator of Rab small GTPases, which orchestrate the intracellular membrane trafficking involved in cell proliferation. Deletion mutants of Dd rbg-3 exhibited an increased growth rate and a shortened developmental period, while an overexpression mutant demonstrated the opposite effects. We also show that Dd Rbg-3 interacts with 2 Gα subunits in an activity-dependent manner in vitro. Furthermore, both human and Caenorhabditis elegans rbg-3 homologs complemented the Dd rbg-3-deletion phenotype in D. discoideum, indicating that similar pathways may be generally conserved in multicellular organisms.
Conclusions/significance: Our findings suggest that Dd Rbg-3 acts as a key element regulating the duration of D. discoideum life-span potentially via trimeric G-protein cascades.
Conflict of interest statement
Figures
Similar articles
-
Trafficking of adhesion and aggregation-modulating proteins during the early stages of Dictyostelium development.Cell Signal. 2024 Sep;121:111292. doi: 10.1016/j.cellsig.2024.111292. Epub 2024 Jul 8. Cell Signal. 2024. PMID: 38986731
-
CbfA, the C-module DNA-binding factor, plays an essential role in the initiation of Dictyostelium discoideum development.Eukaryot Cell. 2004 Oct;3(5):1349-58. doi: 10.1128/EC.3.5.1349-1358.2004. Eukaryot Cell. 2004. PMID: 15470262 Free PMC article.
-
Loss of cAMP-specific phosphodiesterase rescues spore development in G protein mutant in dictyostelium.Cell Signal. 2014 Feb;26(2):409-18. doi: 10.1016/j.cellsig.2013.10.003. Cell Signal. 2014. PMID: 24511612 Free PMC article.
-
Rho GTPase signaling in Dictyostelium discoideum: insights from the genome.Eur J Cell Biol. 2006 Sep;85(9-10):947-59. doi: 10.1016/j.ejcb.2006.04.011. Epub 2006 Jun 8. Eur J Cell Biol. 2006. PMID: 16762450 Review.
-
Genes involved in Dictyostelium discoideum sexual reproduction.Eur J Cell Biol. 2006 Sep;85(9-10):961-8. doi: 10.1016/j.ejcb.2006.05.012. Epub 2006 Jul 11. Eur J Cell Biol. 2006. PMID: 16815590 Review.
Cited by
-
Deletion of gmfA induces keratocyte-like migration in Dictyostelium.FEBS Open Bio. 2022 Jan;12(1):306-319. doi: 10.1002/2211-5463.13339. Epub 2021 Dec 12. FEBS Open Bio. 2022. PMID: 34855306 Free PMC article.
-
A striatal-enriched intronic GPCR modulates huntingtin levels and toxicity.Elife. 2015 Mar 4;4:e05449. doi: 10.7554/eLife.05449. Elife. 2015. PMID: 25738228 Free PMC article.
-
Glutathione S-transferase 4 is a putative DIF-binding protein that regulates the size of fruiting bodies in Dictyostelium discoideum.Biochem Biophys Rep. 2016 Sep 19;8:219-226. doi: 10.1016/j.bbrep.2016.09.006. eCollection 2016 Dec. Biochem Biophys Rep. 2016. PMID: 28955959 Free PMC article.
References
-
- Frasa MA, Koessmeier KT, Ahmadian MR, Braga VM (2012) Illuminating the functional and structural repertoire of human TBC/RABGAPs. Nat Rev Mol Cell Biol 13: 67–73. - PubMed
-
- Ginsburg GT, Gollop R, Yu Y, Louis JM, Saxe CL, et al. (1995) The regulation of Dictyostelium development by transmembrane signaling. J Eukaryot Microbiol 42: 200–205. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

