Aluminum induces cross-resistance of potato to Phytophthora infestans
- PMID: 24346311
- PMCID: PMC3928512
- DOI: 10.1007/s00425-013-2008-8
Aluminum induces cross-resistance of potato to Phytophthora infestans
Abstract
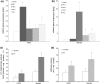
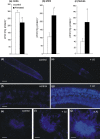
The phenomenon of cross-resistance allows plants to acquire resistance to a broad range of stresses after previous exposure to one specific factor. Although this stress-response relationship has been known for decades, the sequence of events that underpin cross-resistance remains unknown. Our experiments revealed that susceptible potato (Solanum tuberosum L. cv. Bintje) undergoing aluminum (Al) stress at the root level showed enhanced defense responses correlated with reduced disease symptoms after leaf inoculation with Phytophthora infestans. The protection capacity of Al to subsequent stress was associated with the local accumulation of H2O2 in roots and systemic activation of salicylic acid (SA) and nitric oxide (NO) dependent pathways. The most crucial Al-mediated changes involved coding of NO message in an enhanced S-nitrosothiol formation in leaves tuned with an abundant SNOs accumulation in the main vein of leaves. Al-induced distal NO generation was correlated with the overexpression of PR-2 and PR-3 at both mRNA and protein activity levels. In turn, after contact with a pathogen we observed early up-regulation of SA-mediated defense genes, e.g. PR1, PR-2, PR-3 and PAL, and subsequent disease limitation. Taken together Al exposure induced distal changes in the biochemical stress imprint, facilitating more effective responses to a subsequent pathogen attack.
Figures

Similar articles
-
Normoergic NO-dependent changes, triggered by a SAR inducer in potato, create more potent defense responses to Phytophthora infestans.Plant Sci. 2013 Oct;211:23-34. doi: 10.1016/j.plantsci.2013.06.007. Epub 2013 Jun 21. Plant Sci. 2013. PMID: 23987808
-
The combined nitrate reductase and nitrite-dependent route of NO synthesis in potato immunity to Phytophthora infestans.Plant Physiol Biochem. 2016 Nov;108:468-477. doi: 10.1016/j.plaphy.2016.08.009. Epub 2016 Aug 18. Plant Physiol Biochem. 2016. PMID: 27588710
-
Contrasting Regulation of NO and ROS in Potato Defense-Associated Metabolism in Response to Pathogens of Different Lifestyles.PLoS One. 2016 Oct 3;11(10):e0163546. doi: 10.1371/journal.pone.0163546. eCollection 2016. PLoS One. 2016. PMID: 27695047 Free PMC article.
-
The potato transcription factor StbZIP61 regulates dynamic biosynthesis of salicylic acid in defense against Phytophthora infestans infection.Plant J. 2018 Sep;95(6):1055-1068. doi: 10.1111/tpj.14010. Epub 2018 Jul 20. Plant J. 2018. PMID: 29952082
-
Curdlan β-1,3-glucooligosaccharides induce the defense responses against Phytophthora infestans infection of potato (Solanum tuberosum L. cv. McCain G1) leaf cells.PLoS One. 2014 May 9;9(5):e97197. doi: 10.1371/journal.pone.0097197. eCollection 2014. PLoS One. 2014. PMID: 24816730 Free PMC article.
Cited by
-
S-Nitrosoglutathione Reductase-The Master Regulator of Protein S-Nitrosation in Plant NO Signaling.Plants (Basel). 2019 Feb 21;8(2):48. doi: 10.3390/plants8020048. Plants (Basel). 2019. PMID: 30795534 Free PMC article. Review.
-
Insight into metabolic sensors of nitrosative stress protection in Phytophthora infestans.Front Plant Sci. 2023 Jul 20;14:1148222. doi: 10.3389/fpls.2023.1148222. eCollection 2023. Front Plant Sci. 2023. PMID: 37546259 Free PMC article.
-
An update on redox signals in plant responses to biotic and abiotic stress crosstalk: insights from cadmium and fungal pathogen interactions.J Exp Bot. 2021 Aug 11;72(16):5857-5875. doi: 10.1093/jxb/erab271. J Exp Bot. 2021. PMID: 34111283 Free PMC article. Review.
-
How plants respond to heavy metal contamination: a narrative review of proteomic studies and phytoremediation applications.Planta. 2024 Mar 29;259(5):103. doi: 10.1007/s00425-024-04378-2. Planta. 2024. PMID: 38551683 Review.
-
Aluminum, a Friend or Foe of Higher Plants in Acid Soils.Front Plant Sci. 2017 Oct 12;8:1767. doi: 10.3389/fpls.2017.01767. eCollection 2017. Front Plant Sci. 2017. PMID: 29075280 Free PMC article. Review.
References
-
- Achuo EA, Prinsen E, Höfte M. Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathol. 2006;55:178–186. doi: 10.1111/j.1365-3059.2006.01340.x. - DOI
-
- Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001;24:1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. - DOI
-
- Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Deckert J, Rucińska-Sobkowiak R, Gzyl J, Pawlak-Sprada S, Abramowski D, Jelonek T, Gwóźdź EA. Nitric oxide implication in cadmium-induced programmed cell death in roots and signaling response of yellow lupine plants. Plant Physiol Biochem. 2012;58:124–134. doi: 10.1016/j.plaphy.2012.06.018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

