Trying to understand gestational diabetes
- PMID: 24341419
- PMCID: PMC4178541
- DOI: 10.1111/dme.12381
Trying to understand gestational diabetes
Abstract
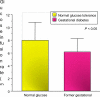
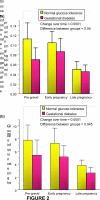
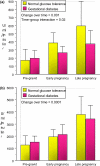
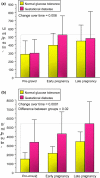
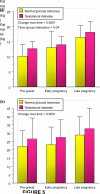
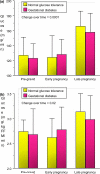
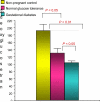
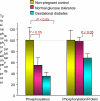
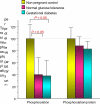
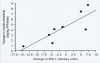
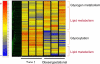
Women with normal glucose tolerance pre-gravid and developing gestational diabetes in late gestation have subclinical metabolic dysfunction prior to conception compared with women with normal glucose tolerance. Because of the 60% decrease in insulin sensitivity with normal pregnancy, these women develop clinical hyperglycaemia/gestational diabetes in late gestation. The metabolic dysfunction includes impaired insulin response, decreased hepatic suppression of glucose production during insulin infusion and decreased insulin-stimulated glucose uptake in skeletal muscle, i.e. peripheral insulin resistance. The insulin resistance in normal glucose tolerance pregnancy is related to a decrease in the post-receptor insulin signalling cascade, specifically decreased insulin receptor substrate 1 tyrosine phosphorylation. In women with normal glucose tolerance this is reversed post-partum. In contrast, in gestational diabetes, in addition to the decrease in insulin receptor substrate 1 tyrosine phosphorylation, there is an additional decrease in tyrosine phosphorylation of the intracellular portion of the insulin receptor that is not related to the insulin receptor protein content. Post-partum women with gestational diabetes, who had retention of gestational weight gain, had no significant improvement in insulin sensitivity and increased inflammation expressed as increased plasma and skeletal muscle tumour necrosis factor alpha. The increased inflammation or meta-inflammation is a hallmark of obesity and during pregnancy develops in both white adipose tissue and placenta. Last gene array studies of placenta were associated with alterations in gene expression relating primarily to lipid in contrast to glucose metabolic pathways in gestational diabetes compared with Type 1 diabetes. Future studies are directed at decreasing inflammation prior to and during pregnancy using various lifestyle and nutritional interventions.
© 2013 The Author. Diabetic Medicine © 2013 Diabetes UK.
Figures

Similar articles
-
Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes.Diabetes. 1999 Sep;48(9):1807-14. doi: 10.2337/diabetes.48.9.1807. Diabetes. 1999. PMID: 10480612
-
Vanadate enhances but does not normalize glucose transport and insulin receptor phosphorylation in skeletal muscle from obese women with gestational diabetes mellitus.Am J Obstet Gynecol. 2000 Nov;183(5):1263-70. doi: 10.1067/mob.2000.106816. Am J Obstet Gynecol. 2000. PMID: 11084576
-
Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum.Diabetes. 2008 Mar;57(3):606-13. doi: 10.2337/db07-1356. Epub 2007 Dec 14. Diabetes. 2008. PMID: 18083784 Free PMC article.
-
Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance.Dan Med J. 2014 Jul;61(7):B4890. Dan Med J. 2014. PMID: 25123125 Review.
-
Gestational diabetes mellitus and subsequent development of overt diabetes mellitus.Dan Med Bull. 1998 Nov;45(5):495-509. Dan Med Bull. 1998. PMID: 9850811 Review.
Cited by
-
Exploring preconception signatures of metabolites in mothers with gestational diabetes mellitus using a non-targeted approach.BMC Med. 2023 Mar 16;21(1):99. doi: 10.1186/s12916-023-02819-5. BMC Med. 2023. PMID: 36927416 Free PMC article.
-
Research Gaps in Gestational Diabetes Mellitus: Executive Summary of a National Institute of Diabetes and Digestive and Kidney Diseases Workshop.Obstet Gynecol. 2018 Aug;132(2):496-505. doi: 10.1097/AOG.0000000000002726. Obstet Gynecol. 2018. PMID: 29995731 Free PMC article.
-
The fetal glucose steal: an underappreciated phenomenon in diabetic pregnancy.Diabetologia. 2016 Jun;59(6):1089-94. doi: 10.1007/s00125-016-3931-6. Epub 2016 Mar 19. Diabetologia. 2016. PMID: 26995651 Free PMC article. Review.
-
The Role of HIV Infection in the Pathophysiology of Gestational Diabetes Mellitus and Hypertensive Disorders of Pregnancy.Front Cardiovasc Med. 2021 May 12;8:613930. doi: 10.3389/fcvm.2021.613930. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34055923 Free PMC article. Review.
-
Healthy dietary patterns and common pregnancy complications: a prospective and longitudinal study.Am J Clin Nutr. 2021 Sep 1;114(3):1229-1237. doi: 10.1093/ajcn/nqab145. Am J Clin Nutr. 2021. PMID: 34075392 Free PMC article.
References
-
- Catalano PM, Bernstein IM, Wolfe RR, Srikanta S, Tyzbir E, Sims EAH. Subclinical abnormalities of glucose metabolism in subjects with previous gestational diabetes. Am J Obstet Gynecol. 1986;155:1255–1262. - PubMed
-
- DeFronzo R, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:214–223. - PubMed
-
- Catalano PM, Tyzbir ED, Wolfe RR, Calles J, Roman NM, Amini SB, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264:E60–E67. - PubMed
-
- Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obest Gynecol. 1999;180:903–916. - PubMed
-
- Catalano PM, Roman-Drago NM, Amini SB, Sims EAH. Longitudinal changes in body composition and energy balance in lean women with normal and abnormal glucose tolerance during pregnancy. Am J Obstet Gynecol. 1998;179:156–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

