Label retention identifies a multipotent mesenchymal stem cell-like population in the postnatal thymus
- PMID: 24340075
- PMCID: PMC3858364
- DOI: 10.1371/journal.pone.0083024
Label retention identifies a multipotent mesenchymal stem cell-like population in the postnatal thymus
Abstract
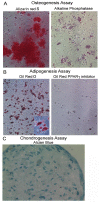
Thymic microenvironments are essential for the proper development and selection of T cells critical for a functional and self-tolerant adaptive immune response. While significant turnover occurs, it is unclear whether populations of adult stem cells contribute to the maintenance of postnatal thymic epithelial microenvironments. Here, the slow cycling characteristic of stem cells and their property of label-retention were used to identify a K5-expressing thymic stromal cell population capable of generating clonal cell lines that retain the capacity to differentiate into a number of mesenchymal lineages including adipocytes, chondrocytes and osteoblasts suggesting a mesenchymal stem cell-like phenotype. Using cell surface analysis both culture expanded LRCs and clonal thymic mesenchymal cell lines were found to express Sca1, PDGFRα, PDGFRβ,CD29, CD44, CD49F, and CD90 similar to MSCs. Sorted GFP-expressing stroma, that give rise to TMSC lines, contribute to thymic architecture when reaggregated with fetal stroma and transplanted under the kidney capsule of nude mice. Together these results show that the postnatal thymus contains a population of mesenchymal stem cells that can be maintained in culture and suggests they may contribute to the maintenance of functional thymic microenvironments.
Conflict of interest statement
Figures



Similar articles
-
Isolation and phenotypical characterization of mesenchymal stem cells from human fetal thymus.Bull Exp Biol Med. 2005 Jan;139(1):134-40. doi: 10.1007/s10517-005-0231-4. Bull Exp Biol Med. 2005. PMID: 16142296
-
A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells.Stem Cell Res Ther. 2016 Jul 28;7(1):97. doi: 10.1186/s13287-016-0359-3. Stem Cell Res Ther. 2016. PMID: 27465541 Free PMC article.
-
Characterization and histological localization of multipotent mesenchymal stromal cells in the human postnatal thymus.Stem Cells Dev. 2008 Dec;17(6):1165-74. doi: 10.1089/scd.2007.0252. Stem Cells Dev. 2008. PMID: 19006457
-
The biology of equine mesenchymal stem cells: phenotypic characterization, cell surface markers and multilineage differentiation.Front Biosci (Landmark Ed). 2012 Jan 1;17(3):892-908. doi: 10.2741/3963. Front Biosci (Landmark Ed). 2012. PMID: 22201780 Review.
-
Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges.Histol Histopathol. 2013 Sep;28(9):1109-16. doi: 10.14670/HH-28.1109. Epub 2013 Apr 16. Histol Histopathol. 2013. PMID: 23588700 Free PMC article. Review.
Cited by
-
Restoration of Thymus Function with Bioengineered Thymus Organoids.Curr Stem Cell Rep. 2016 Jun;2(2):128-139. doi: 10.1007/s40778-016-0040-x. Curr Stem Cell Rep. 2016. PMID: 27529056 Free PMC article.
-
Cutting to the chase: taking the pulse of label-retaining cells in kidney.Am J Physiol Renal Physiol. 2015 Jan 1;308(1):F29-30. doi: 10.1152/ajprenal.00538.2014. Epub 2014 Oct 8. Am J Physiol Renal Physiol. 2015. PMID: 25298529 Free PMC article. No abstract available.
-
Revelations in Thymic Epithelial Cell Biology and Heterogeneity from Single-Cell RNA Sequencing and Lineage Tracing Methodologies.Methods Mol Biol. 2023;2580:25-49. doi: 10.1007/978-1-0716-2740-2_2. Methods Mol Biol. 2023. PMID: 36374449 Free PMC article.
-
Bone Marrow-Derived Cells Contribute to the Maintenance of Thymic Stroma including TECs.J Immunol Res. 2022 Apr 29;2022:6061746. doi: 10.1155/2022/6061746. eCollection 2022. J Immunol Res. 2022. PMID: 35528618 Free PMC article.
-
Twenty Years of AIRE.Front Immunol. 2018 Feb 12;9:98. doi: 10.3389/fimmu.2018.00098. eCollection 2018. Front Immunol. 2018. PMID: 29483906 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA137788/CA/NCI NIH HHS/United States
- AI059739/AI/NIAID NIH HHS/United States
- R01 AI059739/AI/NIAID NIH HHS/United States
- R01 AI083988/AI/NIAID NIH HHS/United States
- G12 MD007603/MD/NIMHD NIH HHS/United States
- G12 RR003060/RR/NCRR NIH HHS/United States
- SC1 AI081527/AI/NIAID NIH HHS/United States
- SC1 AI104994/AI/NIAID NIH HHS/United States
- 8G12MD007603-27/MD/NIMHD NIH HHS/United States
- 1SC1AI081527/AI/NIAID NIH HHS/United States
- 9SC1AI104994/AI/NIAID NIH HHS/United States
- 2G12RR03060-26A1/RR/NCRR NIH HHS/United States
- 1U54CA137788/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

