Innate immune response induced by baculovirus attenuates transgene expression in mammalian cells
- PMID: 24335288
- PMCID: PMC3911546
- DOI: 10.1128/JVI.03055-13
Innate immune response induced by baculovirus attenuates transgene expression in mammalian cells
Abstract
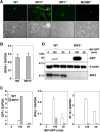
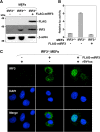
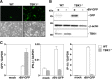
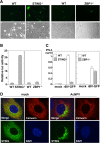
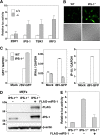
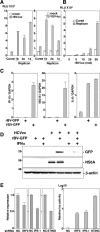
The baculovirus Autographa californica nucleopolyhedrovirus (AcNPV) has been widely used to achieve a high level of foreign gene expression in insect cells, as well as for efficient gene transduction into mammalian cells without any replication. In addition to permitting efficient gene delivery, baculovirus has been shown to induce host innate immune responses in various mammalian cells and in mice. In this study, we examined the effects of the innate immune responses on gene expression by recombinant baculoviruses in cultured cells. The reporter gene expression in IRF3-deficient mouse embryonic fibroblasts (MEFs) infected with the recombinant baculovirus was shown to be enhanced in accordance with the suppression of beta interferon (IFN-β) production. Furthermore, efficient gene transduction by the recombinant baculovirus was achieved in MEFs deficient for stimulator of interferon genes (STING), TANK binding kinase 1 (TBK1), IFN regulatory factor 3 (IRF3), or IFN-β promoter stimulator 1 (IPS-1), but not in those deficient for IRF7, MyD88, or Z-DNA binding protein 1 (ZBP1)/DAI. Enhancement of gene expression by the recombinant baculovirus was also observed in human hepatoma cell lines replicating hepatitis C virus (HCV), in which innate immunity was impaired by the cleavage of IPS-1 by the viral protease. In addition, infection with the recombinant baculovirus expressing the BH3-only protein, BIMS, a potent inducer of apoptosis, resulted in a selective cell death in the HCV replicon cells. These results indicate that innate immune responses induced by infection with baculovirus attenuate transgene expression, and this characteristic might be useful for a selective gene transduction into cells with impaired innate immunity arising from infection with various viruses.
Figures

Similar articles
-
Baculovirus Transduction in Mammalian Cells Is Affected by the Production of Type I and III Interferons, Which Is Mediated Mainly by the cGAS-STING Pathway.J Virol. 2020 Oct 14;94(21):e01555-20. doi: 10.1128/JVI.01555-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32796076 Free PMC article.
-
Baculovirus induces type I interferon production through toll-like receptor-dependent and -independent pathways in a cell-type-specific manner.J Virol. 2009 Aug;83(15):7629-40. doi: 10.1128/JVI.00679-09. Epub 2009 May 27. J Virol. 2009. PMID: 19474102 Free PMC article.
-
[Analysis of the application of host innate immune response to control and prevent infection].Uirusu. 2012 Jun;62(1):103-12. doi: 10.2222/jsv.62.103. Uirusu. 2012. PMID: 23189830 Review. Japanese.
-
Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus.J Virol. 2005 Mar;79(5):2847-58. doi: 10.1128/JVI.79.5.2847-2858.2005. J Virol. 2005. PMID: 15709004 Free PMC article.
-
Baculovirus as a Tool for Gene Delivery and Gene Therapy.Viruses. 2018 Sep 19;10(9):510. doi: 10.3390/v10090510. Viruses. 2018. PMID: 30235841 Free PMC article. Review.
Cited by
-
Effectiveness of different avian influenza (H5) vaccination regimens in layer chickens on the humoral immune response and interferon-alpha signalling immune marker.Vet Res Commun. 2018 Jun;42(2):145-152. doi: 10.1007/s11259-018-9717-1. Epub 2018 Apr 4. Vet Res Commun. 2018. PMID: 29619666
-
Baculovirus Transduction in Mammalian Cells Is Affected by the Production of Type I and III Interferons, Which Is Mediated Mainly by the cGAS-STING Pathway.J Virol. 2020 Oct 14;94(21):e01555-20. doi: 10.1128/JVI.01555-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32796076 Free PMC article.
-
Apoptosis dysfunction: unravelling the interplay between ZBP1 activation and viral invasion in innate immune responses.Cell Commun Signal. 2024 Feb 24;22(1):149. doi: 10.1186/s12964-024-01531-y. Cell Commun Signal. 2024. PMID: 38402193 Free PMC article. Review.
-
Synthetic Virus-Derived Nanosystems (SVNs) for Delivery and Precision Docking of Large Multifunctional DNA Circuitry in Mammalian Cells.Pharmaceutics. 2020 Aug 11;12(8):759. doi: 10.3390/pharmaceutics12080759. Pharmaceutics. 2020. PMID: 32796680 Free PMC article. Review.
-
Role of tumor suppressor genes in the cancer-associated reprogramming of human induced pluripotent stem cells.Stem Cell Res Ther. 2014;5(2):58. doi: 10.1186/scrt447. Stem Cell Res Ther. 2014. PMID: 25157408 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

