RhoA modulates signaling through the mechanistic target of rapamycin complex 1 (mTORC1) in mammalian cells
- PMID: 24316235
- PMCID: PMC3951878
- DOI: 10.1016/j.cellsig.2013.11.035
RhoA modulates signaling through the mechanistic target of rapamycin complex 1 (mTORC1) in mammalian cells
Abstract
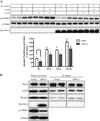
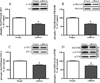
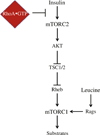
The mechanistic target of rapamycin (mTOR) in complex 1 (mTORC1) pathway integrates signals generated by hormones and nutrients to control cell growth and metabolism. The activation state of mTORC1 is regulated by a variety of GTPases including Rheb and Rags. Recently, Rho1, the yeast ortholog of RhoA, was shown to interact directly with TORC1 and repress its activation state in yeast. Thus, the purpose of the present study was to test the hypothesis that the RhoA GTPase modulates signaling through mTORC1 in mammalian cells. In support of this hypothesis, exogenous overexpression of either wild type or constitutively active (ca)RhoA repressed mTORC1 signaling as assessed by phosphorylation of p70S6K1 (Thr389), 4E-BP1 (Ser65) and ULK1 (Ser757). Additionally, RhoA·GTP repressed phosphorylation of mTORC1-associated mTOR (Ser2481). The RhoA·GTP mediated repression of mTORC1 signaling occurred independent of insulin or leucine induced stimulation. In contrast to the action of Rho1 in yeast, no evidence was found to support a direct interaction of RhoA·GTP with mTORC1. Instead, expression of caRheb, but not caRags, was able to rescue the RhoA·GTP mediated repression of mTORC1 suggesting RhoA functions upstream of Rheb to repress mTORC1 activity. Consistent with this suggestion, RhoA·GTP repressed phosphorylation of TSC2 (Ser939), PRAS40 (Thr246), Akt (Ser473), and mTORC2-associated mTOR (Ser2481). Overall, the results support a model in which RhoA·GTP represses mTORC1 signaling upstream of Akt and mTORC2.
Keywords: 4E-BP1; 70kDa ribosomal protein S6 kinase 1; Akt; PRAS40; RAPTOR; RAPTOR-independent companion of mTOR; RICTOR; Sin1; TSC; TSC2; ULK1; UTR; caRags; caRheb; caRhoA; constitutively active Rags B and C; constitutively active Rheb; constitutively active RhoA; eukaryotic initiation factor 4E binding protein 1; mTOR; mTORC; mTORC2; mechanistic target of rapamycin; mechanistic target of rapamycin complex; p70S6K1; proline-rich Akt/PKB substrate 40kDa; regulatory-associated protein of mTOR, complex 1; stress-activated map kinase-interacting protein 1; tuberous sclerosis complex; uncoordinated-51-like kinase 1; untranslated region; wild type RhoA; wtRhoA.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Receptor-recognized α₂-macroglobulin binds to cell surface-associated GRP78 and activates mTORC1 and mTORC2 signaling in prostate cancer cells.PLoS One. 2012;7(12):e51735. doi: 10.1371/journal.pone.0051735. Epub 2012 Dec 14. PLoS One. 2012. PMID: 23272152 Free PMC article.
-
Alkaline intracellular pH (pHi) increases PI3K activity to promote mTORC1 and mTORC2 signaling and function during growth factor limitation.J Biol Chem. 2023 Sep;299(9):105097. doi: 10.1016/j.jbc.2023.105097. Epub 2023 Jul 26. J Biol Chem. 2023. PMID: 37507012 Free PMC article.
-
The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1.J Biol Chem. 2007 Jul 13;282(28):20329-39. doi: 10.1074/jbc.M702636200. Epub 2007 May 21. J Biol Chem. 2007. PMID: 17517883 Free PMC article.
-
Amino acid regulation of TOR complex 1.Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E592-602. doi: 10.1152/ajpendo.90645.2008. Epub 2008 Sep 2. Am J Physiol Endocrinol Metab. 2009. PMID: 18765678 Free PMC article. Review.
-
Regulation of mTORC1 by PI3K signaling.Trends Cell Biol. 2015 Sep;25(9):545-55. doi: 10.1016/j.tcb.2015.06.002. Epub 2015 Jul 6. Trends Cell Biol. 2015. PMID: 26159692 Free PMC article. Review.
Cited by
-
Genetics, environmental stress, and amino acid supplementation affect lactational performance via mTOR signaling pathway in bovine mammary epithelial cells.Front Genet. 2023 Aug 10;14:1195774. doi: 10.3389/fgene.2023.1195774. eCollection 2023. Front Genet. 2023. PMID: 37636261 Free PMC article. Review.
-
REDD2 expression in rat skeletal muscle correlates with nutrient-induced activation of mTORC1: responses to aging, immobilization, and remobilization.Am J Physiol Endocrinol Metab. 2015 Jan 15;308(2):E122-9. doi: 10.1152/ajpendo.00341.2014. Epub 2014 Nov 18. Am J Physiol Endocrinol Metab. 2015. PMID: 25406262 Free PMC article.
-
Low RhoA expression is associated with adverse outcome in melanoma patients: a clinicopathological analysis.Am J Transl Res. 2019 Jul 15;11(7):4524-4532. eCollection 2019. Am J Transl Res. 2019. PMID: 31396356 Free PMC article.
-
RhoA G17V is sufficient to induce autoimmunity and promotes T-cell lymphomagenesis in mice.Blood. 2018 Aug 30;132(9):935-947. doi: 10.1182/blood-2017-11-818617. Epub 2018 May 16. Blood. 2018. PMID: 29769264 Free PMC article.
-
Reduced REDD1 expression contributes to activation of mTORC1 following electrically induced muscle contraction.Am J Physiol Endocrinol Metab. 2014 Oct 15;307(8):E703-11. doi: 10.1152/ajpendo.00250.2014. Epub 2014 Aug 26. Am J Physiol Endocrinol Metab. 2014. PMID: 25159324 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

