Insights into redox sensing metalloproteins in Mycobacterium tuberculosis
- PMID: 24314844
- PMCID: PMC3959581
- DOI: 10.1016/j.jinorgbio.2013.11.003
Insights into redox sensing metalloproteins in Mycobacterium tuberculosis
Abstract
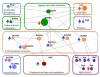
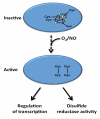
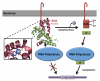
Mycobacterium tuberculosis, the pathogen that causes tuberculosis, has evolved sophisticated mechanisms for evading assault by the human host. This review focuses on M. tuberculosis regulatory metalloproteins that are sensitive to exogenous stresses attributed to changes in the levels of gaseous molecules (i.e., molecular oxygen, carbon monoxide and nitric oxide) to elicit an intracellular response. In particular, we highlight recent developments on the subfamily of Whi proteins, redox sensing WhiB-like proteins that contain iron-sulfur clusters, sigma factors and their cognate anti-sigma factors of which some are zinc-regulated, and the dormancy survival regulon DosS/DosT-DosR heme sensory system. Mounting experimental evidence suggests that these systems contribute to a highly complex and interrelated regulatory network that controls M. tuberculosis biology. This review concludes with a discussion of strategies that M. tuberculosis has developed to maintain redox homeostasis, including mechanisms to regulate endogenous nitric oxide and carbon monoxide levels.
Keywords: Hypoxia; Metalloproteins; Molecular gas sensing; Mycobacterium tuberculosis; Redox sensing.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
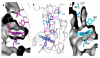
Similar articles
-
Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor.Proc Natl Acad Sci U S A. 2007 Jul 10;104(28):11568-73. doi: 10.1073/pnas.0705054104. Epub 2007 Jul 3. Proc Natl Acad Sci U S A. 2007. PMID: 17609369 Free PMC article.
-
The mechanism of redox sensing in Mycobacterium tuberculosis.Free Radic Biol Med. 2012 Oct 15;53(8):1625-41. doi: 10.1016/j.freeradbiomed.2012.08.008. Epub 2012 Aug 11. Free Radic Biol Med. 2012. PMID: 22921590 Review.
-
Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon.J Biol Chem. 2008 Jun 27;283(26):18032-9. doi: 10.1074/jbc.M802274200. Epub 2008 Apr 9. J Biol Chem. 2008. PMID: 18400743 Free PMC article.
-
DosT and DevS are oxygen-switched kinases in Mycobacterium tuberculosis.Protein Sci. 2007 Aug;16(8):1708-19. doi: 10.1110/ps.072897707. Epub 2007 Jun 28. Protein Sci. 2007. PMID: 17600145 Free PMC article.
-
Redox biology of tuberculosis pathogenesis.Adv Microb Physiol. 2012;60:263-324. doi: 10.1016/B978-0-12-398264-3.00004-8. Adv Microb Physiol. 2012. PMID: 22633061 Review.
Cited by
-
Multiplex quantitative SILAC for analysis of archaeal proteomes: a case study of oxidative stress responses.Environ Microbiol. 2018 Jan;20(1):385-401. doi: 10.1111/1462-2920.14014. Epub 2017 Dec 29. Environ Microbiol. 2018. PMID: 29194950 Free PMC article.
-
Fe-S proteins that regulate gene expression.Biochim Biophys Acta. 2015 Jun;1853(6):1284-93. doi: 10.1016/j.bbamcr.2014.11.018. Epub 2014 Nov 20. Biochim Biophys Acta. 2015. PMID: 25450978 Free PMC article. Review.
-
Heme catabolism in the causative agent of anthrax.Mol Microbiol. 2019 Aug;112(2):515-531. doi: 10.1111/mmi.14270. Epub 2019 May 27. Mol Microbiol. 2019. PMID: 31063630 Free PMC article.
-
Host-pathogen redox dynamics modulate Mycobacterium tuberculosis pathogenesis.Pathog Dis. 2018 Jul 1;76(5):fty036. doi: 10.1093/femspd/fty036. Pathog Dis. 2018. PMID: 29873719 Free PMC article.
-
Oxidative Unfolding of the Rubredoxin Domain and the Natively Disordered N-terminal Region Regulate the Catalytic Activity of Mycobacterium tuberculosis Protein Kinase G.J Biol Chem. 2016 Dec 30;291(53):27062-27072. doi: 10.1074/jbc.M116.747089. Epub 2016 Nov 3. J Biol Chem. 2016. PMID: 27810897 Free PMC article.
References
-
- W.H. Organization 2012.
-
- Wayne LG, Sohaskey CD. Annu Rev Microbiol. 2001;55:139–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

