New viruses for cancer therapy: meeting clinical needs
- PMID: 24292552
- PMCID: PMC4002503
- DOI: 10.1038/nrmicro3140
New viruses for cancer therapy: meeting clinical needs
Abstract
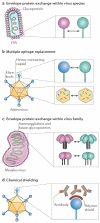
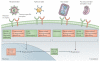
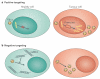
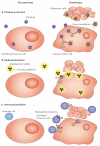
Early-stage clinical trials of oncolytic virotherapy have reported the safety of several virus platforms, and viruses from three families have progressed to advanced efficacy trials. In addition, preclinical studies have established proof-of-principle for many new genetic engineering strategies. Thus, the virotherapy field now has available a diverse collection of viruses that are equipped to address unmet clinical needs owing to improved systemic administration, greater tumour specificity and enhanced oncolytic efficacy. The current key challenge for the field is to develop viruses that replicate with greater efficiency within tumours while achieving therapeutic synergy with currently available treatments.
Figures
Similar articles
-
Introduction to Oncolytic Virotherapy.Methods Mol Biol. 2020;2058:1-6. doi: 10.1007/978-1-4939-9794-7_1. Methods Mol Biol. 2020. PMID: 31486028 Review.
-
Optimization and preclinical design of genetically engineered viruses for human oncolytic therapy.Expert Opin Biol Ther. 2012 Nov;12(11):1427-47. doi: 10.1517/14712598.2012.707183. Epub 2012 Jul 13. Expert Opin Biol Ther. 2012. PMID: 22788715 Review.
-
Oncolytic viruses in the treatment of cancer: a review of current strategies.Pathol Oncol Res. 2012 Oct;18(4):771-81. doi: 10.1007/s12253-012-9548-2. Epub 2012 Jun 20. Pathol Oncol Res. 2012. PMID: 22714538 Review.
-
Advanced new strategies for metastatic cancer treatment by therapeutic stem cells and oncolytic virotherapy.Oncotarget. 2016 Sep 6;7(36):58684-58695. doi: 10.18632/oncotarget.11017. Oncotarget. 2016. PMID: 27494901 Free PMC article. Review.
-
Oncolytic Virotherapy: An Advanced Microbial Approach for the Management of Cancer.Crit Rev Eukaryot Gene Expr. 2024;34(1):1-13. doi: 10.1615/CritRevEukaryotGeneExpr.2023048962. Crit Rev Eukaryot Gene Expr. 2024. PMID: 37824388
Cited by
-
Nuclear reprogramming with a non-integrating human RNA virus.Stem Cell Res Ther. 2015 Mar 26;6(1):48. doi: 10.1186/s13287-015-0035-z. Stem Cell Res Ther. 2015. PMID: 25889591 Free PMC article.
-
Complement inhibition prevents oncolytic vaccinia virus neutralization in immune humans and cynomolgus macaques.Mol Ther. 2015 Jun;23(6):1066-1076. doi: 10.1038/mt.2015.49. Epub 2015 Mar 25. Mol Ther. 2015. PMID: 25807289 Free PMC article.
-
Coxsackievirus Type B3 Is a Potent Oncolytic Virus against KRAS-Mutant Lung Adenocarcinoma.Mol Ther Oncolytics. 2019 Jul 23;14:266-278. doi: 10.1016/j.omto.2019.07.003. eCollection 2019 Sep 27. Mol Ther Oncolytics. 2019. PMID: 31463367 Free PMC article.
-
White paper on microbial anti-cancer therapy and prevention.J Immunother Cancer. 2018 Aug 6;6(1):78. doi: 10.1186/s40425-018-0381-3. J Immunother Cancer. 2018. PMID: 30081947 Free PMC article. Review.
-
How to train your oncolytic virus: the immunological sequel.Mol Ther. 2014 Nov;22(11):1881-4. doi: 10.1038/mt.2014.188. Mol Ther. 2014. PMID: 25365984 Free PMC article. No abstract available.
References
-
- Dock G. The influence of complicating diseases upon leukemia. Am. J. Med. Sci. 1904;127:563–592.
-
- Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15:651–659. - PubMed
-
This paper provides a narrative introduction to the history of oncolytic virotherapy.
-
- Liu T-C, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nature Rev. Clin. Oncol. 2007;4:101–117. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

