CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI
- PMID: 24285636
- PMCID: PMC4484851
- DOI: 10.1002/cm.21159
CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI
Abstract
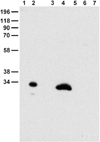
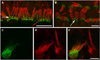
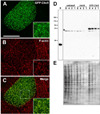
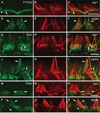
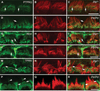
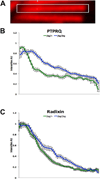
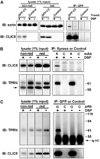
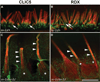
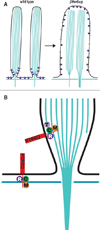
Chloride intracellular channel 5 protein (CLIC5) was originally isolated from microvilli in complex with actin binding proteins including ezrin, a member of the Ezrin-Radixin-Moesin (ERM) family of membrane-cytoskeletal linkers. CLIC5 concentrates at the base of hair cell stereocilia and is required for normal hearing and balance in mice, but its functional significance is poorly understood. This study investigated the role of CLIC5 in postnatal development and maintenance of hair bundles. Confocal and scanning electron microscopy of CLIC5-deficient jitterbug (jbg) mice revealed progressive fusion of stereocilia as early as postnatal day 10. Radixin (RDX), protein tyrosine phosphatase receptor Q (PTPRQ), and taperin (TPRN), deafness-associated proteins that also concentrate at the base of stereocilia, were mislocalized in fused stereocilia of jbg mice. TPRQ and RDX were dispersed even prior to stereocilia fusion. Biochemical assays showed interaction of CLIC5 with ERM proteins, TPRN, and possibly myosin VI (MYO6). In addition, CLIC5 and RDX failed to localize normally in fused stereocilia of MYO6 mutant mice. Based on these findings, we propose a model in which these proteins work together as a complex to stabilize linkages between the plasma membrane and subjacent actin cytoskeleton at the base of stereocilia.
Keywords: PTPRQ; chloride intracellular channel 5 (CLIC5); cytoskeleton; deafness; ezrin-radixin-moesin (ERM); hair cell; myosin VI (MYO6); radixin; stereocilia; taperin.
© Published 2013 Wiley Periodicals, Inc. This article is a US government work and, as such, is in the public domain in the United States of America.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


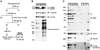
Similar articles
-
The chloride intracellular channel protein CLIC5 is expressed at high levels in hair cell stereocilia and is essential for normal inner ear function.J Neurosci. 2006 Oct 4;26(40):10188-98. doi: 10.1523/JNEUROSCI.2166-06.2006. J Neurosci. 2006. PMID: 17021174 Free PMC article.
-
Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia.J Cell Biol. 2004 Aug 16;166(4):559-70. doi: 10.1083/jcb.200402007. J Cell Biol. 2004. PMID: 15314067 Free PMC article.
-
Tprn is essential for the integrity of stereociliary rootlet in cochlear hair cells in mice.Front Med. 2019 Dec;13(6):690-704. doi: 10.1007/s11684-018-0638-8. Epub 2018 Aug 30. Front Med. 2019. PMID: 30159668
-
CLIC proteins, ezrin, radixin, moesin and the coupling of membranes to the actin cytoskeleton: a smoking gun?Biochim Biophys Acta. 2014 Feb;1838(2):643-57. doi: 10.1016/j.bbamem.2013.05.025. Epub 2013 Jun 1. Biochim Biophys Acta. 2014. PMID: 23732235 Review.
-
Stereocilia morphogenesis and maintenance through regulation of actin stability.Semin Cell Dev Biol. 2017 May;65:88-95. doi: 10.1016/j.semcdb.2016.08.017. Epub 2016 Aug 23. Semin Cell Dev Biol. 2017. PMID: 27565685 Free PMC article. Review.
Cited by
-
Synaptojanin2 Mutation Causes Progressive High-frequency Hearing Loss in Mice.Front Cell Neurosci. 2020 Sep 25;14:561857. doi: 10.3389/fncel.2020.561857. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33100973 Free PMC article.
-
Clinical Characteristics and In Vitro Analysis of MYO6 Variants Causing Late-Onset Progressive Hearing Loss.Genes (Basel). 2020 Mar 4;11(3):273. doi: 10.3390/genes11030273. Genes (Basel). 2020. PMID: 32143290 Free PMC article.
-
Keratan sulfate, an electrosensory neurosentient bioresponsive cell instructive glycosaminoglycan.Glycobiology. 2024 Apr 1;34(3):cwae014. doi: 10.1093/glycob/cwae014. Glycobiology. 2024. PMID: 38376199 Free PMC article. Review.
-
First confirmatory study on PTPRQ as an autosomal dominant non-syndromic hearing loss gene.J Transl Med. 2019 Oct 26;17(1):351. doi: 10.1186/s12967-019-2099-5. J Transl Med. 2019. PMID: 31655630 Free PMC article.
-
Mutation of the EPHA2 Tyrosine-Kinase Domain Dysregulates Cell Pattern Formation and Cytoskeletal Gene Expression in the Lens.Cells. 2021 Sep 30;10(10):2606. doi: 10.3390/cells10102606. Cells. 2021. PMID: 34685586 Free PMC article.
References
-
- Avraham KB, Hasson T, Sobe T, Balsara B, Testa JR, Skvorak AB, Morton CC, Copeland NG, Jenkins NA. Characterization of unconventional MYO6, the human homologue of the gene responsible for deafness in Snell’s waltzer mice. Hum Mol Genet. 1997;6:1225–1231. - PubMed
-
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol. 2005;7:148–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

