Induction of endocycles represses apoptosis independently of differentiation and predisposes cells to genome instability
- PMID: 24284207
- PMCID: PMC3865753
- DOI: 10.1242/dev.098871
Induction of endocycles represses apoptosis independently of differentiation and predisposes cells to genome instability
Abstract
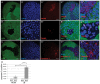
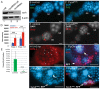
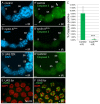
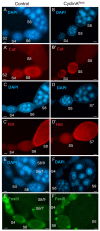
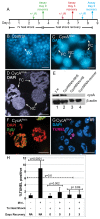
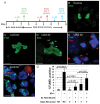
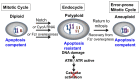
The endocycle is a common developmental cell cycle variation wherein cells become polyploid through repeated genome duplication without mitosis. We previously showed that Drosophila endocycling cells repress the apoptotic cell death response to genotoxic stress. Here, we investigate whether it is differentiation or endocycle remodeling that promotes apoptotic repression. We find that when nurse and follicle cells switch into endocycles during oogenesis they repress the apoptotic response to DNA damage caused by ionizing radiation, and that this repression has been conserved in the genus Drosophila over 40 million years of evolution. Follicle cells defective for Notch signaling failed to switch into endocycles or differentiate and remained apoptotic competent. However, genetic ablation of mitosis by knockdown of Cyclin A or overexpression of fzr/Cdh1 induced follicle cell endocycles and repressed apoptosis independently of Notch signaling and differentiation. Cells recovering from these induced endocycles regained apoptotic competence, showing that repression is reversible. Recovery from fzr/Cdh1 overexpression also resulted in an error-prone mitosis with amplified centrosomes and high levels of chromosome loss and fragmentation. Our results reveal an unanticipated link between endocycles and the repression of apoptosis, with broader implications for how endocycles may contribute to genome instability and oncogenesis.
Keywords: Apoptosis; Cell cycle; Drosophila; Endocycle; Oogenesis.
Figures

Similar articles
-
The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions.Development. 2004 Jul;131(13):3169-81. doi: 10.1242/dev.01172. Epub 2004 Jun 2. Development. 2004. PMID: 15175253
-
Premature endocycling of Drosophila follicle cells causes pleiotropic defects in oogenesis.Genetics. 2024 Apr 3;226(4):iyae009. doi: 10.1093/genetics/iyae009. Genetics. 2024. PMID: 38302115
-
Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells.Development. 2001 Dec;128(23):4737-46. doi: 10.1242/dev.128.23.4737. Development. 2001. PMID: 11731454
-
At the crossroads of differentiation and proliferation: precise control of cell-cycle changes by multiple signaling pathways in Drosophila follicle cells.Bioessays. 2011 Feb;33(2):124-34. doi: 10.1002/bies.201000089. Bioessays. 2011. PMID: 21154780 Free PMC article. Review.
-
Endocycles: a recurrent evolutionary innovation for post-mitotic cell growth.Nat Rev Mol Cell Biol. 2014 Mar;15(3):197-210. doi: 10.1038/nrm3756. Nat Rev Mol Cell Biol. 2014. PMID: 24556841 Review.
Cited by
-
Different cell cycle modifications repress apoptosis at different steps independent of developmental signaling in Drosophila.Mol Biol Cell. 2016 Jun 15;27(12):1885-97. doi: 10.1091/mbc.E16-03-0139. Epub 2016 Apr 13. Mol Biol Cell. 2016. PMID: 27075174 Free PMC article.
-
Extracellular matrix stiffness cues junctional remodeling for 3D tissue elongation.Nat Commun. 2019 Jul 26;10(1):3339. doi: 10.1038/s41467-019-10874-x. Nat Commun. 2019. PMID: 31350387 Free PMC article.
-
Developmentally Programmed Switches in DNA Replication: Gene Amplification and Genome-Wide Endoreplication in Tetrahymena.Microorganisms. 2023 Feb 16;11(2):491. doi: 10.3390/microorganisms11020491. Microorganisms. 2023. PMID: 36838456 Free PMC article.
-
Wound-induced polyploidization is driven by Myc and supports tissue repair in the presence of DNA damage.Development. 2019 Aug 2;146(15):dev173005. doi: 10.1242/dev.173005. Development. 2019. PMID: 31315896 Free PMC article.
-
An unscheduled switch to endocycles induces a reversible senescent arrest that impairs growth of the Drosophila wing disc.PLoS Genet. 2024 Sep 3;20(9):e1011387. doi: 10.1371/journal.pgen.1011387. eCollection 2024 Sep. PLoS Genet. 2024. PMID: 39226333 Free PMC article.
References
-
- Bachtrog D., Thornton K., Clark A., Andolfatto P. (2006). Extensive introgression of mitochondrial DNA relative to nuclear genes in the Drosophila yakuba species group. Evolution 60, 292–302 - PubMed
-
- Bartkova J., Rezaei N., Liontos M., Karakaidos P., Kletsas D., Issaeva N., Vassiliou L. V., Kolettas E., Niforou K., Zoumpourlis V. C., et al. (2006). Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637 - PubMed
-
- Boveri T. (2008). Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J. Cell Sci. 121 Suppl., S1–S84 - PubMed
-
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

