The Sybtraps: control of synaptobrevin traffic by synaptophysin, α-synuclein and AP-180
- PMID: 24279465
- PMCID: PMC3992847
- DOI: 10.1111/tra.12140
The Sybtraps: control of synaptobrevin traffic by synaptophysin, α-synuclein and AP-180
Abstract
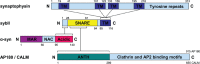
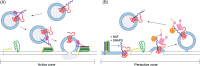
Synaptobrevin II (sybII) is a key fusogenic molecule on synaptic vesicles (SVs) therefore the active maintenance of both its conformation and location in sufficient numbers on this organelle is critical in both mediating and sustaining neurotransmitter release. Recently three proteins have been identified having key roles in the presentation, trafficking and retrieval of sybII during the fusion and endocytosis of SVs. The nerve terminal protein α-synuclein catalyses sybII entry into SNARE complexes, whereas the monomeric adaptor protein AP-180 is required for sybII retrieval during SV endocytosis. Overarching these events is the tetraspan SV protein synaptophysin, which is a major sybII interaction partner on the SV. This review will evaluate recent studies to propose working models for the control of sybII traffic by synaptophysin and other Sybtraps (sybII trafficking partners) and suggest how dysfunction in sybII traffic may contribute to human disease.
Keywords: AP-180; endocytosis; presynapse; synaptobrevin; synaptophysin; vesicle; α-synuclein.
© 2013 The Authors. Traffic published by John Wiley & Sons Ltd.
Figures
Similar articles
-
The iTRAPs: Guardians of Synaptic Vesicle Cargo Retrieval During Endocytosis.Front Synaptic Neurosci. 2016 Feb 9;8:1. doi: 10.3389/fnsyn.2016.00001. eCollection 2016. Front Synaptic Neurosci. 2016. PMID: 26903854 Free PMC article. Review.
-
Synaptophysin sustains presynaptic performance by preserving vesicular synaptobrevin-II levels.J Neurochem. 2019 Oct;151(1):28-37. doi: 10.1111/jnc.14797. Epub 2019 Jul 16. J Neurochem. 2019. PMID: 31216055 Free PMC article.
-
A Fine Balance of Synaptophysin Levels Underlies Efficient Retrieval of Synaptobrevin II to Synaptic Vesicles.PLoS One. 2016 Feb 12;11(2):e0149457. doi: 10.1371/journal.pone.0149457. eCollection 2016. PLoS One. 2016. PMID: 26871701 Free PMC article.
-
Synaptophysin controls synaptobrevin-II retrieval via a cryptic C-terminal interaction site.J Biol Chem. 2021 Jan-Jun;296:100266. doi: 10.1016/j.jbc.2021.100266. Epub 2021 Jan 8. J Biol Chem. 2021. PMID: 33769286 Free PMC article.
-
Synaptophysin-dependent synaptobrevin-2 trafficking at the presynapse-Mechanism and function.J Neurochem. 2021 Oct;159(1):78-89. doi: 10.1111/jnc.15499. Epub 2021 Sep 9. J Neurochem. 2021. PMID: 34468992 Review.
Cited by
-
Inhibition of STAT3 signal pathway recovers postsynaptic plasticity to improve cognitive impairment caused by chronic intermittent hypoxia.Sleep Breath. 2023 Jun;27(3):893-902. doi: 10.1007/s11325-022-02671-6. Epub 2022 Jul 23. Sleep Breath. 2023. PMID: 35871214
-
Cellular function and pathological role of ATP13A2 and related P-type transport ATPases in Parkinson's disease and other neurological disorders.Front Mol Neurosci. 2014 May 27;7:48. doi: 10.3389/fnmol.2014.00048. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24904274 Free PMC article. Review.
-
ATP binding to synaspsin IIa regulates usage and clustering of vesicles in terminals of hippocampal neurons.J Neurosci. 2015 Jan 21;35(3):985-98. doi: 10.1523/JNEUROSCI.0944-14.2015. J Neurosci. 2015. PMID: 25609616 Free PMC article.
-
Neurons, Glia, Extracellular Matrix and Neurovascular Unit: A Systems Biology Approach to the Complexity of Synaptic Plasticity in Health and Disease.Int J Mol Sci. 2020 Feb 24;21(4):1539. doi: 10.3390/ijms21041539. Int J Mol Sci. 2020. PMID: 32102370 Free PMC article. Review.
-
The iTRAPs: Guardians of Synaptic Vesicle Cargo Retrieval During Endocytosis.Front Synaptic Neurosci. 2016 Feb 9;8:1. doi: 10.3389/fnsyn.2016.00001. eCollection 2016. Front Synaptic Neurosci. 2016. PMID: 26903854 Free PMC article. Review.
References
-
- Jung N, Haucke V. Clathrin-mediated endocytosis at synapses. Traffic. 2007;8:1129–1136. - PubMed
-
- Kelly BT, Owen DJ. Endocytic sorting of transmembrane protein cargo. Curr Opin Cell Biol. 2011;23:404–412. - PubMed
-
- Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. - PubMed
-
- Diril MK, Wienisch M, Jung N, Klingauf J, Haucke V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell. 2006;10:233–244. - PubMed
-
- Koo SJ, Markovic S, Puchkov D, Mahrenholz CC, Beceren-Braun F, Maritzen T, Dernedde J, Volkmer R, Oschkinat H, Haucke V. SNARE motif-mediated sorting of synaptobrevin by the endocytic adaptors clathrin assembly lymphoid myeloid leukemia (CALM) and AP180 at synapses. Proc Natl Acad Sci USA. 2011;108:13540–13545. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

