Both GLS silencing and GLS2 overexpression synergize with oxidative stress against proliferation of glioma cells
- PMID: 24276018
- PMCID: PMC4327995
- DOI: 10.1007/s00109-013-1105-2
Both GLS silencing and GLS2 overexpression synergize with oxidative stress against proliferation of glioma cells
Abstract
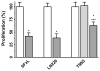
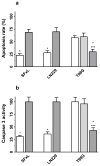
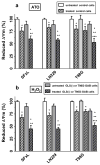
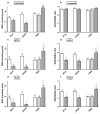
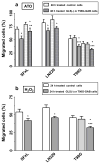
Mitochondrial glutaminase (GA) plays an essential role in cancer cell metabolism, contributing to biosynthesis, bioenergetics, and redox balance. Humans contain several GA isozymes encoded by the GLS and GLS2 genes, but the specific roles of each in cancer metabolism are still unclear. In this study, glioma SFxL and LN229 cells with silenced isoenzyme glutaminase KGA (encoded by GLS) showed lower survival ratios and a reduced GSH-dependent antioxidant capacity. These GLS-silenced cells also demonstrated induction of apoptosis indicated by enhanced annexin V binding capacity and caspase 3 activity. GLS silencing was associated with decreased mitochondrial membrane potential (ΔΨm) (JC-1 dye test), indicating that apoptosis was mediated by mitochondrial dysfunction. Similar observations were made in T98 glioma cells overexpressing glutaminase isoenzyme GAB, encoded by GLS2, though some characteristics (GSH/GSSG ratio) were different in the differently treated cell lines. Thus, control of GA isoenzyme expression may prove to be a key tool to alter both metabolic and oxidative stress in cancer therapy. Interestingly, reactive oxygen species (ROS) generation by treatment with oxidizing agents: arsenic trioxide or hydrogen peroxide, synergizes with either KGA silencing or GAB overexpression to suppress malignant properties of glioma cells, including the reduction of cellular motility. Of note, negative modulation of GLS isoforms or GAB overexpression evoked lower c-myc and bcl-2 expression, as well as higher pro-apoptotic bid expression. Combination of modulation of GA expression and treatment with oxidizing agents may become a therapeutic strategy for intractable cancers and provides a multi-angle evaluation system for anti-glioma pre-clinical investigations.
Key message: Silencing GLS or overexpressing GLS2 induces growth inhibition in glioma cell lines. Inhibition is synergistically enhanced after arsenic trioxide (ATO) or H2O2 treatment. Glutatione levels decrease in GLS-silenced cells but augment if GLS2 is overexpressed. ROS synergistically inhibit cell migration by GLS silencing or GLS2 overexpression. c-myc, bid, and bcl-2 mediate apoptosis resulting from GLS silencing or GLS2 overexpression.
Conflict of interest statement
Figures




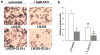
Similar articles
-
Glutaminase isoforms expression switches microRNA levels and oxidative status in glioblastoma cells.J Biomed Sci. 2021 Feb 20;28(1):14. doi: 10.1186/s12929-021-00712-y. J Biomed Sci. 2021. PMID: 33610185 Free PMC article.
-
Opposing roles of glutaminase isoforms in determining glioblastoma cell phenotype.Neurochem Int. 2015 Sep;88:6-9. doi: 10.1016/j.neuint.2014.11.004. Epub 2014 Dec 18. Neurochem Int. 2015. PMID: 25529918 Review.
-
Silencing of GLS and overexpression of GLS2 genes cooperate in decreasing the proliferation and viability of glioblastoma cells.Tumour Biol. 2014 Mar;35(3):1855-62. doi: 10.1007/s13277-013-1247-4. Epub 2013 Oct 6. Tumour Biol. 2014. PMID: 24096582 Free PMC article.
-
Transfection with GLS2 Glutaminase (GAB) Sensitizes Human Glioblastoma Cell Lines to Oxidative Stress by a Common Mechanism Involving Suppression of the PI3K/AKT Pathway.Cancers (Basel). 2019 Jan 19;11(1):115. doi: 10.3390/cancers11010115. Cancers (Basel). 2019. PMID: 30669455 Free PMC article.
-
Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer.Curr Mol Med. 2013 May;13(4):514-34. doi: 10.2174/1566524011313040005. Curr Mol Med. 2013. PMID: 22934847 Review.
Cited by
-
GLS2 Is a Tumor Suppressor and a Regulator of Ferroptosis in Hepatocellular Carcinoma.Cancer Res. 2022 Sep 16;82(18):3209-3222. doi: 10.1158/0008-5472.CAN-21-3914. Cancer Res. 2022. PMID: 35895807 Free PMC article.
-
Targeting Glutamine Addiction in Gliomas.Cancers (Basel). 2020 Jan 29;12(2):310. doi: 10.3390/cancers12020310. Cancers (Basel). 2020. PMID: 32013066 Free PMC article. Review.
-
Glutaminase inhibitor compound 968 inhibits cell proliferation and sensitizes paclitaxel in ovarian cancer.Am J Transl Res. 2016 Oct 15;8(10):4265-4277. eCollection 2016. Am J Transl Res. 2016. PMID: 27830010 Free PMC article.
-
CircCOL1A1 promotes proliferation, migration, and invasion of colorectal cancer (CRC) cells and glutamine metabolism through GLS1 up-regulation by sponging miR-214-3p.J Cancer Res Clin Oncol. 2024 Apr 25;150(4):211. doi: 10.1007/s00432-024-05736-z. J Cancer Res Clin Oncol. 2024. PMID: 38662258 Free PMC article.
-
Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/beta-catenin pathway.EBioMedicine. 2019 Jan;39:239-254. doi: 10.1016/j.ebiom.2018.11.063. Epub 2018 Dec 13. EBioMedicine. 2019. PMID: 30555042 Free PMC article.
References
-
- Márquez J, de la Oliva AR, Matés JM, Segura JA, Alonso FJ. Glutaminase: a multifaceted protein not only involved in generating glutamate. Neurochem Int. 2006;48:465–471. - PubMed
-
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. - PubMed
-
- Olalla L, Gutiérrez A, Campos JA, Khan ZU, Alonso FJ, Segura JA, Márquez J, Aledo JC. Nuclear localization of L-type glutaminase in mammalian brain. J Biol Chem. 2002;277:38939–38944. - PubMed
-
- Márquez J, Tosina M, de la Rosa V, Segura JA, Alonso FJ, Matés JM, Campos-Sandoval JA. New insights into brain glutaminases: beyond their role on glutamatergic transmission. Neurochem Int. 2009;55:64–70. - PubMed
-
- Dang CV. MYC, microRNAs and glutamine addiction in cancers. Cell Cycle. 2009;8:3243–3245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

