Chimeric antigen receptor therapy for cancer
- PMID: 24274181
- PMCID: PMC4120077
- DOI: 10.1146/annurev-med-060512-150254
Chimeric antigen receptor therapy for cancer
Abstract
Improved outcomes for patients with cancer hinge on the development of new targeted therapies with acceptable short-term and long-term toxicity. Progress in basic, preclinical, and clinical arenas spanning cellular immunology, synthetic biology, and cell-processing technologies has paved the way for clinical applications of chimeric antigen receptor-based therapies. This new form of targeted immunotherapy merges the exquisite targeting specificity of monoclonal antibodies with the potent cytotoxicity and long-term persistence provided by cytotoxic T cells. Although this field is still in its infancy, clinical trials have already shown clinically significant antitumor activity in neuroblastoma, chronic lymphocytic leukemia, and B cell lymphoma, and trials targeting a variety of other adult and pediatric malignancies are under way. Ongoing work is focused on identifying optimal tumor targets and on elucidating and manipulating both cell- and host-associated factors to support expansion and persistence of the genetically engineered cells in vivo. The potential to target essentially any tumor-associated cell-surface antigen for which a monoclonal antibody can be made opens up an entirely new arena for targeted therapy of cancer.
Conflict of interest statement
The University of Pennsylvania has entered into a partnership with Novartis for the development of chimeric antigen receptors. This partnership is managed in accordance with the University of Pennsylvania’s Conflict of Interest Policy. All authors are in compliance with this policy.
Figures
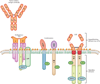

Similar articles
-
The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer.Clin Cancer Res. 2012 May 15;18(10):2780-90. doi: 10.1158/1078-0432.CCR-11-1920. Clin Cancer Res. 2012. PMID: 22589486 Free PMC article.
-
Chimeric antigen receptor engineering: a right step in the evolution of adoptive cellular immunotherapy.Int Rev Immunol. 2015 Mar;34(2):154-87. doi: 10.3109/08830185.2015.1018419. Int Rev Immunol. 2015. PMID: 25901860 Review.
-
Prospects for personalized combination immunotherapy for solid tumors based on adoptive cell therapies and immune checkpoint blockade therapies.Nihon Rinsho Meneki Gakkai Kaishi. 2017;40(1):68-77. doi: 10.2177/jsci.40.68. Nihon Rinsho Meneki Gakkai Kaishi. 2017. PMID: 28539557 Review.
-
Chimeric Antigen Receptor T Cell Therapy in Hematology.Turk J Haematol. 2015 Dec;32(4):285-94. doi: 10.4274/tjh.2015.0049. Epub 2015 Aug 6. Turk J Haematol. 2015. PMID: 26377367 Free PMC article. Review.
-
[T Lymphocytes with Modified Specificity in the Therapy of Malignant Diseases].Mol Biol (Mosk). 2017 Nov-Dec;51(6):1008-1023. doi: 10.7868/S002689841706012X. Mol Biol (Mosk). 2017. PMID: 29271964 Review. Russian.
Cited by
-
Genetic Manipulation of NK Cells for Cancer Immunotherapy: Techniques and Clinical Implications.Front Immunol. 2015 Jun 10;6:266. doi: 10.3389/fimmu.2015.00266. eCollection 2015. Front Immunol. 2015. PMID: 26113846 Free PMC article. Review.
-
Messenger RNA encoding constitutively active Toll-like receptor 4 enhances effector functions of human T cells.Clin Exp Immunol. 2015 Nov;182(2):220-9. doi: 10.1111/cei.12688. Epub 2015 Aug 28. Clin Exp Immunol. 2015. PMID: 26212048 Free PMC article.
-
Targeting the tumor vasculature to enhance T cell activity.Curr Opin Immunol. 2015 Apr;33:55-63. doi: 10.1016/j.coi.2015.01.011. Epub 2015 Feb 6. Curr Opin Immunol. 2015. PMID: 25665467 Free PMC article. Review.
-
Oncogene addiction: pathways of therapeutic response, resistance, and road maps toward a cure.EMBO Rep. 2015 Mar;16(3):280-96. doi: 10.15252/embr.201439949. Epub 2015 Feb 13. EMBO Rep. 2015. PMID: 25680965 Free PMC article. Review.
-
Treatment of allergic eosinophilic asthma through engineered IL-5-anchored chimeric antigen receptor T cells.Cell Discov. 2022 Aug 16;8(1):80. doi: 10.1038/s41421-022-00433-y. Cell Discov. 2022. PMID: 35973984 Free PMC article.
References
-
- Barnes DW, Ford CE, Ilbery PL, et al. Tissue transplantation in the radiation chimera. J. Cell. Physiol. Suppl. 1957;50:123–138. - PubMed
-
- Barnes DW, Loutit JF. Treatment of murine leukaemia with x-rays and homologous bone marrow. II. Br. J. Haematol. 1957;3:241–252. - PubMed
-
- Mathe G, Amiel JL, Schwarzenberg L, et al. Adoptive immunotherapy of acute leukemia: experimental and clinical results. Cancer Res. 1965;25:1525–1531. - PubMed
-
- Rosenberg SA, Terry WD. Passive immunotherapy of cancer in animals and man. Adv. Cancer Res. 1977;25:323–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

