Deficiency of zebrafish fgf20a results in aberrant skull remodeling that mimics both human cranial disease and evolutionarily important fish skull morphologies
- PMID: 24261444
- PMCID: PMC3890419
- DOI: 10.1111/ede.12052
Deficiency of zebrafish fgf20a results in aberrant skull remodeling that mimics both human cranial disease and evolutionarily important fish skull morphologies
Abstract
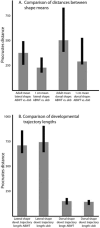
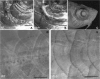
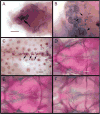
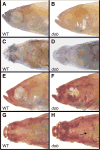
The processes that direct skull remodeling are of interest to both human-oriented studies of cranial dysplasia and evolutionary studies of skull divergence. There is increasing awareness that these two fields can be mutually informative when natural variation mimics pathology. Here we describe a zebrafish mutant line, devoid of blastema (dob), which does not have a functional fgf20a protein, and which also presents cranial defects similar to both adaptive and clinical variation. We used geometric morphometric methods to provide quantitative descriptions of the effects of the dob mutation on skull morphogenesis. In combination with "whole-mount in situ hybridization" labeling of normal fgf20a expression and assays for osteoblast and osteoclast activity, the results of these analyses indicate that cranial dysmorphologies in dob zebrafish are generated by aberrations in post-embryonic skull remodeling via decreased osteoblasotgenesis and increased osteoclastogenesis. Mutational effects include altered skull vault geometries and midfacial hypoplasia that are consistent with key diagnostic signs for multiple human craniofacial syndromes. These phenotypic shifts also mimic changes in the functional morphology of fish skulls that have arisen repeatedly in several highly successful radiations (e.g., damselfishes and East-African rift-lake cichlids). Our results offer the dob/fgf20a mutant as an experimentally tractable model with which to examine post-embryonic skull development as it relates to human disease and vertebrate evolution.
© 2013 Wiley Periodicals, Inc.
Figures


Similar articles
-
fgf20 is essential for initiating zebrafish fin regeneration.Science. 2005 Dec 23;310(5756):1957-60. doi: 10.1126/science.1117637. Science. 2005. PMID: 16373575
-
Fgfr3 Is a Positive Regulator of Osteoblast Expansion and Differentiation During Zebrafish Skull Vault Development.J Bone Miner Res. 2020 Sep;35(9):1782-1797. doi: 10.1002/jbmr.4042. Epub 2020 May 26. J Bone Miner Res. 2020. PMID: 32379366
-
Osteoclast activity sculpts craniofacial form to permit sensorineural patterning in the zebrafish skull.Front Endocrinol (Lausanne). 2022 Nov 1;13:969481. doi: 10.3389/fendo.2022.969481. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36387889 Free PMC article.
-
Zebrafish Craniofacial Development: A Window into Early Patterning.Curr Top Dev Biol. 2015;115:235-69. doi: 10.1016/bs.ctdb.2015.07.001. Epub 2015 Oct 6. Curr Top Dev Biol. 2015. PMID: 26589928 Free PMC article. Review.
-
Craniofacial dysostosis syndromes: evaluation and staged reconstructive approach.Atlas Oral Maxillofac Surg Clin North Am. 2010 Sep;18(2):109-28. doi: 10.1016/j.cxom.2010.08.006. Atlas Oral Maxillofac Surg Clin North Am. 2010. PMID: 21036313 Review. No abstract available.
Cited by
-
Expression variations in ectodysplasin-A gene (eda) may contribute to morphological divergence of scales in haplochromine cichlids.BMC Ecol Evol. 2022 Mar 10;22(1):28. doi: 10.1186/s12862-022-01984-0. BMC Ecol Evol. 2022. PMID: 35272610 Free PMC article.
-
Thyroid hormone modulation during zebrafish development recapitulates evolved diversity in danionin jaw protrusion mechanics.Evol Dev. 2019 Sep;21(5):231-246. doi: 10.1111/ede.12299. Epub 2019 Aug 2. Evol Dev. 2019. PMID: 31374588 Free PMC article.
-
Fish is Fish: the use of experimental model species to reveal causes of skeletal diversity in evolution and disease.J Appl Ichthyol. 2014 Aug 1;30(4):616-629. doi: 10.1111/jai.12533. J Appl Ichthyol. 2014. PMID: 25221374 Free PMC article.
-
Conserved but flexible modularity in the zebrafish skull: implications for craniofacial evolvability.Proc Biol Sci. 2018 Apr 25;285(1877):20172671. doi: 10.1098/rspb.2017.2671. Proc Biol Sci. 2018. PMID: 29669899 Free PMC article.
-
Ciliary Rootlet Coiled-Coil 2 (crocc2) Is Associated with Evolutionary Divergence and Plasticity of Cichlid Jaw Shape.Mol Biol Evol. 2021 Jul 29;38(8):3078-3092. doi: 10.1093/molbev/msab071. Mol Biol Evol. 2021. PMID: 33720362 Free PMC article.
References
-
- Adams CE, Fraser D, Huntingford FA, Greer RB, Askew CM, Walker AF. Trophic polymorphism amongst Arctic charr from Loch Rannoch, Scotland. Journal of Fish Biology. 1998;52:1259–1271.
-
- Adams DC, Otárola-Castillo E. geomorph: an r package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution. 2013;4:393–399.
-
- Albertson RC, Yelick PC. Zebrafish: 2nd Edition Cellular and Developmental Biology. Elsevier Academic Press Inc; San Diego: 2004. Morphogenesis of the jaw: Development beyond the embryo; pp. 437–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

