Ubiquitin-specific proteases 25 negatively regulates virus-induced type I interferon signaling
- PMID: 24260525
- PMCID: PMC3832446
- DOI: 10.1371/journal.pone.0080976
Ubiquitin-specific proteases 25 negatively regulates virus-induced type I interferon signaling
Abstract
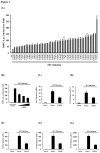
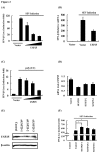
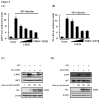
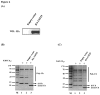
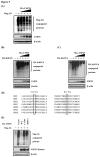
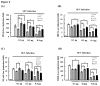
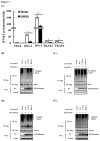
Ubiquitination and deubiquitination have emerged as critical regulatory processes in the virus-triggered type I interferon (IFN) induction pathway. In this study, we carried out a targeted siRNA screen of 54 ubiquitin-specific proteases (USPs) and identified USP25 as a negative regulator of the virus-triggered type I IFN signaling pathway. Overexpression of USP25 inhibited virus-induced activation of IFN-β, interferon regulation factor 3 (IRF3) and nuclear factor-kappa B (NF-κB), as well as the phosphorylation of IRF3 and NF-κB subunit p65. Furthermore, Knockdown of USP25 potentiated virus-induced induction of the IFN-β. In addition, detailed analysis demonstrated that USP25 cleaved lysine 48- and lysine 63-linked polyubiquitin chains in vitro and in vivo, and its deubiquitinating enzyme (DUB) activity, were dependent on a cysteine residue (Cys178) and a histidine residue (His607). USP25 mutants lacking DUB activity lost the ability to block virus-induced type I IFN to some degree. Mechanistically, USP25 deubiquitinated retinoic acid-inducible gene I (RIG-I), tumornecrosis factor (TNF) receptor-associated factor 2 (TRAF2), and TRAF6 to inhibit RIG-I-like receptor-mediated IFN signaling. Our findings suggest that USP25 is a novel DUB negatively regulating virus-induced type I IFN production.
Conflict of interest statement
Figures
Similar articles
-
Phosphatase Cdc25A Negatively Regulates the Antiviral Immune Response by Inhibiting TBK1 Activity.J Virol. 2018 Sep 12;92(19):e01118-18. doi: 10.1128/JVI.01118-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021902 Free PMC article.
-
Chloroquine attenuates lipopolysaccharide-induced inflammatory responses through upregulation of USP25.Can J Physiol Pharmacol. 2017 May;95(5):481-491. doi: 10.1139/cjpp-2016-0303. Epub 2016 Nov 30. Can J Physiol Pharmacol. 2017. PMID: 28134560
-
Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFα-induced cancer cell migration.Biochem J. 2012 Feb 1;441(3):979-86. doi: 10.1042/BJ20111358. Biochem J. 2012. PMID: 22029577
-
Structure and function of the highly homologous deubiquitinases ubiquitin specific peptidase 25 and 28: Insights into their pathophysiological and therapeutic roles.Biochem Pharmacol. 2023 Jul;213:115624. doi: 10.1016/j.bcp.2023.115624. Epub 2023 May 26. Biochem Pharmacol. 2023. PMID: 37245535 Review.
-
The lncRNAs involved in regulating the RIG-I signaling pathway.Front Cell Infect Microbiol. 2022 Nov 9;12:1041682. doi: 10.3389/fcimb.2022.1041682. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36439216 Free PMC article. Review.
Cited by
-
Ubiquitin signaling in immune responses.Cell Res. 2016 Apr;26(4):457-83. doi: 10.1038/cr.2016.40. Epub 2016 Mar 25. Cell Res. 2016. PMID: 27012466 Free PMC article. Review.
-
UCHL3 promotes ovarian cancer progression by stabilizing TRAF2 to activate the NF-κB pathway.Oncogene. 2020 Jan;39(2):322-333. doi: 10.1038/s41388-019-0987-z. Epub 2019 Sep 2. Oncogene. 2020. PMID: 31477831
-
Inhibitory feedback control of NF-κB signalling in health and disease.Biochem J. 2021 Jul 16;478(13):2619-2664. doi: 10.1042/BCJ20210139. Biochem J. 2021. PMID: 34269817 Free PMC article. Review.
-
Ubiquitin-specific Protease 15 Negatively Regulates Virus-induced Type I Interferon Signaling via Catalytically-dependent and -independent Mechanisms.Sci Rep. 2015 Jun 10;5:11220. doi: 10.1038/srep11220. Sci Rep. 2015. PMID: 26061460 Free PMC article.
-
[Research progress on ubiquitin-specific protease in antiviral immunity].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015 Sep;44(5):578-83. doi: 10.3785/j.issn.1008-9292.2015.09.17. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015. PMID: 26713535 Free PMC article. Review. Chinese.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

