Fluoxetine competes with cortisol for binding to human serum albumin
- PMID: 24250455
- PMCID: PMC3813104
Fluoxetine competes with cortisol for binding to human serum albumin
Abstract
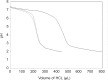
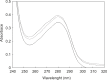
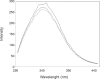
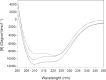
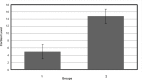
Human serum albumin (HSA) is an important protein that carries variety of substances like some hormones and drugs in blood. Pharmacological studies of the interaction of many drugs and HSA are reported during several decades, specially recently years. Interaction of cortisol and fluoxetine hydrochloride (FLX) (as a common anti-stress drug) with HSA (as their carrier in blood) has been studied separately by using different spectroscopic techniques. Here, considering the increment of anti-stress drugs consumption, conformational change of HSA in presence of cortisol and FLX in 50 mM tris buffer, at pH = 7.5 and 37°C, is investigated via pH meter, UV absorption and fluorescence spectroscopy and circular dichroism methods. pH meter findings indicate that the acid denaturation of HSA in the presence of drug and cortisol occurs in the similar manner and this pattern is different relative to the denaturation of HSA in the absence of two reagents. The results of the other techniques consistent with the pH meter findings show that FLX effects on the physiochemical properties of HSA are as that of Cortisol. In-vivo study in Rats confirms in-vitro findings which means blood cortisol level increased in the presence of FLX. Experimental results indicate that FLX and cortisol alter the structural aspects of HSA in similar manner, so, this findings lead to the following reasonable conclusion: "FLX is a competitive ligand for the binding of cortisol to HSA. Binding of FLX to HSA interferes to the interaction of cortisol-HSA."
Keywords: Cortisol; Fluoxetine; Human serum albumin; Spectroscopic methods; pH meter.
Figures
Similar articles
-
Spectroscopic and molecular modeling study on the separate and simultaneous bindings of alprazolam and fluoxetine hydrochloride to human serum albumin (HSA): with the aim of the drug interactions probing.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 25;137:1106-19. doi: 10.1016/j.saa.2014.08.149. Epub 2014 Oct 7. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25300043
-
Probing the binding of fluoxetine hydrochloride to human serum albumin by multispectroscopic techniques.Spectrochim Acta A Mol Biomol Spectrosc. 2010 Jan;75(1):314-9. doi: 10.1016/j.saa.2009.10.031. Epub 2009 Oct 30. Spectrochim Acta A Mol Biomol Spectrosc. 2010. PMID: 19932052
-
Conformational study of human serum albumin in pre-denaturation temperatures by differential scanning calorimetry, circular dichroism and UV spectroscopy.J Biochem Mol Biol. 2006 Sep 30;39(5):530-6. doi: 10.5483/bmbrep.2006.39.5.530. J Biochem Mol Biol. 2006. PMID: 17002873
-
Probing the interaction of a coumarin-di(2-picolyl)amine hybrid drug-like molecular entity with human serum albumin: Multiple spectroscopic and molecular modeling techniques.Spectrochim Acta A Mol Biomol Spectrosc. 2019 Dec 5;223:117330. doi: 10.1016/j.saa.2019.117330. Epub 2019 Jul 1. Spectrochim Acta A Mol Biomol Spectrosc. 2019. PMID: 31280128
-
Unraveling the versatility of human serum albumin - A comprehensive review of its biological significance and therapeutic potential.Curr Res Struct Biol. 2023 Nov 27;6:100114. doi: 10.1016/j.crstbi.2023.100114. eCollection 2023. Curr Res Struct Biol. 2023. PMID: 38111902 Free PMC article. Review.
Cited by
-
Boosting Neurogenesis in the Adult Hippocampus Using Antidepressants and Mesenchymal Stem Cells.Cells. 2022 Oct 14;11(20):3234. doi: 10.3390/cells11203234. Cells. 2022. PMID: 36291101 Free PMC article. Review.
-
Glucose and fluoxetine induce fine structural change in human serum albumin.Iran J Pharm Res. 2013 Winter;12(1):185-91. Iran J Pharm Res. 2013. PMID: 24250587 Free PMC article.
-
Toxicokinetics of the Antidepressant Fluoxetine and Its Active Metabolite Norfluoxetine in Caenorhabditis elegans and Their Comparative Potency.Environ Sci Technol. 2024 Feb 11;58(7):3129-40. doi: 10.1021/acs.est.3c07744. Online ahead of print. Environ Sci Technol. 2024. PMID: 38343161 Free PMC article.
-
Noncompetitive Inhibition of Bovine Liver Catalase by Lawsone: Kinetics, Binding Mechanism and in silico Modeling Approaches.Iran J Pharm Res. 2020 Winter;19(1):383-397. doi: 10.22037/ijpr.2019.111600.13255. Iran J Pharm Res. 2020. PMID: 32922495 Free PMC article.
-
Introducing Transthyretin as a Differentially Expressed Protein in Washing Subtype of Obsessive-Compulsive Disorder.Basic Clin Neurosci. 2018 May-Jun;9(3):187-194. doi: 10.29252/NIRP.BCN.9.3.187. Basic Clin Neurosci. 2018. PMID: 30034649 Free PMC article.
References
-
- Muller WE, Wollert U. Human serum albumin as a ‘silent receptor’ for drugs and endogenous substances. Pharmacol. 1979;19:56–59. - PubMed
-
- Zhang L, Wang K, Zhang X. Study of the interactions between fluoroquin-olones and human serum albumin by affinity capillary. Electrophoresis Fluorescence Method Anal. Chim. 2007;603:101–110. - PubMed
-
- Liu M, Zhu L, Qu X, Sun D, Li L, Lin R. Studies on the binding of paeonol and two of its isomers to human serum albumin by using microcalorimetry and circular dichroism. J. Chem. Thermodyn. 2007;39:1565–1570.
-
- Ya-Bei H, Shi-Jun W, Yan-Qin Z, Zhang Y, Xiao-Yan G, Ying-Cai T, Da-Ming Z. Investigation on the interaction of prulifloxacin with human serum albumin: a spectroscopic analysis. Chem. Pharm. Bull. 2010;58:582–586. - PubMed
LinkOut - more resources
Full Text Sources
