A potent and selective peptide blocker of the Kv1.3 channel: prediction from free-energy simulations and experimental confirmation
- PMID: 24244345
- PMCID: PMC3820677
- DOI: 10.1371/journal.pone.0078712
A potent and selective peptide blocker of the Kv1.3 channel: prediction from free-energy simulations and experimental confirmation
Abstract
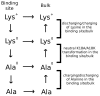
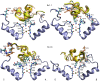
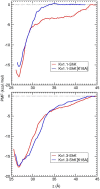
The voltage-gated potassium channel Kv1.3 is a well-established target for treatment of autoimmune diseases. ShK peptide from a sea anemone is one of the most potent blockers of Kv1.3 but its application as a therapeutic agent for autoimmune diseases is limited by its lack of selectivity against other Kv channels, in particular Kv1.1. Accurate models of Kv1.x-ShK complexes suggest that specific charge mutations on ShK could considerably enhance its specificity for Kv1.3. Here we evaluate the K18A mutation on ShK, and calculate the change in binding free energy associated with this mutation using the path-independent free energy perturbation and thermodynamic integration methods, with a novel implementation that avoids convergence problems. To check the accuracy of the results, the binding free energy differences were also determined from path-dependent potential of mean force calculations. The two methods yield consistent results for the K18A mutation in ShK and predict a 2 kcal/mol gain in Kv1.3/Kv1.1 selectivity free energy relative to wild-type peptide. Functional assays confirm the predicted selectivity gain for ShK[K18A] and suggest that it will be a valuable lead in the development of therapeutics for autoimmune diseases.
Conflict of interest statement
Figures
Similar articles
-
N-Terminally extended analogues of the K⁺ channel toxin from Stichodactyla helianthus as potent and selective blockers of the voltage-gated potassium channel Kv1.3.FEBS J. 2015 Jun;282(12):2247-59. doi: 10.1111/febs.13294. Epub 2015 Apr 23. FEBS J. 2015. PMID: 25864722 Free PMC article.
-
A potent and Kv1.3-selective analogue of the scorpion toxin HsTX1 as a potential therapeutic for autoimmune diseases.Sci Rep. 2014 Mar 28;4:4509. doi: 10.1038/srep04509. Sci Rep. 2014. PMID: 24676092 Free PMC article.
-
A C-terminally amidated analogue of ShK is a potent and selective blocker of the voltage-gated potassium channel Kv1.3.FEBS Lett. 2012 Nov 16;586(22):3996-4001. doi: 10.1016/j.febslet.2012.09.038. Epub 2012 Oct 9. FEBS Lett. 2012. PMID: 23063513 Free PMC article.
-
The voltage-gated potassium channel KV1.3 as a therapeutic target for venom-derived peptides.Biochem Pharmacol. 2020 Nov;181:114146. doi: 10.1016/j.bcp.2020.114146. Epub 2020 Jul 10. Biochem Pharmacol. 2020. PMID: 32653588 Review.
-
Analogs of the sea anemone potassium channel blocker ShK for the treatment of autoimmune diseases.Inflamm Allergy Drug Targets. 2011 Oct;10(5):313-21. doi: 10.2174/187152811797200641. Inflamm Allergy Drug Targets. 2011. PMID: 21824083 Free PMC article. Review.
Cited by
-
N-Terminally extended analogues of the K⁺ channel toxin from Stichodactyla helianthus as potent and selective blockers of the voltage-gated potassium channel Kv1.3.FEBS J. 2015 Jun;282(12):2247-59. doi: 10.1111/febs.13294. Epub 2015 Apr 23. FEBS J. 2015. PMID: 25864722 Free PMC article.
-
A Recurrent Motif: Diversity and Evolution of ShKT Domain Containing Proteins in the Vampire Snail Cumia reticulata.Toxins (Basel). 2019 Feb 12;11(2):106. doi: 10.3390/toxins11020106. Toxins (Basel). 2019. PMID: 30759797 Free PMC article.
-
Computational Studies of Venom Peptides Targeting Potassium Channels.Toxins (Basel). 2015 Dec 1;7(12):5194-211. doi: 10.3390/toxins7124877. Toxins (Basel). 2015. PMID: 26633507 Free PMC article. Review.
-
Rapid and accurate structure-based therapeutic peptide design using GPU accelerated thermodynamic integration.Proteins. 2019 Mar;87(3):236-244. doi: 10.1002/prot.25644. Epub 2019 Jan 4. Proteins. 2019. PMID: 30520126 Free PMC article.
-
Long-term obesogenic diet and targeted deletion of potassium channel Kv 1.3 have differing effects on voluntary exercise in mice.Physiol Rep. 2019 Oct;7(20):e14254. doi: 10.14814/phy2.14254. Physiol Rep. 2019. PMID: 31646751 Free PMC article.
References
-
- Castaneda O, Sotolongo V, Amor AM, Stocklin R, Anderson AJ, et al. (1995) Characterization of a potassium channel toxin from the Caribbean Sea anemone Stichodactyla helianthus . Toxicon 33: 603–613. - PubMed
-
- Norton RS, Pennington MW, Wulff H (2004) Potassium channel blockade by the sea anemone toxin ShK for the treatment of multiple sclerosis and other autoimmune diseases. Curr Med Chem 11: 3041–3052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

