AMACO is a component of the basement membrane-associated Fraser complex
- PMID: 24232570
- PMCID: PMC4361737
- DOI: 10.1038/jid.2013.492
AMACO is a component of the basement membrane-associated Fraser complex
Abstract
Fraser syndrome (FS) is a phenotypically variable, autosomal recessive disorder characterized by cryptophthalmus, cutaneous syndactyly, and other malformations resulting from mutations in FRAS1, FREM2, and GRIP1. Transient embryonic epidermal blistering causes the characteristic defects of the disorder. Fras1, Frem1, and Frem2 form the extracellular Fraser complex, which is believed to stabilize the basement membrane. However, several cases of FS could not be attributed to mutations in FRAS1, FREM2, or GRIP1, and FS displays high clinical variability, suggesting that there is an additional genetic, possibly modifying contribution to this disorder. An extracellular matrix protein containing VWA-like domains related to those in matrilins and collagens (AMACO), encoded by the VWA2 gene, has a very similar tissue distribution to the Fraser complex proteins in both mouse and zebrafish. Here, we show that AMACO deposition is lost in Fras1-deficient zebrafish and mice and that Fras1 and AMACO interact directly via their chondroitin sulfate proteoglycan (CSPG) and P2 domains. Knockdown of vwa2, which alone causes no phenotype, enhances the phenotype of hypomorphic Fras1 mutant zebrafish. Together, our data suggest that AMACO represents a member of the Fraser complex.
Conflict of interest statement
The authors state no conflict of interest.
Figures

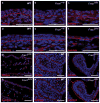
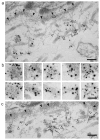
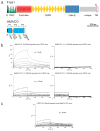


Comment in
-
A new component of the Fraser complex.J Invest Dermatol. 2014 May;134(5):1192-1193. doi: 10.1038/jid.2013.514. J Invest Dermatol. 2014. PMID: 24732331 Free PMC article.
Similar articles
-
A new component of the Fraser complex.J Invest Dermatol. 2014 May;134(5):1192-1193. doi: 10.1038/jid.2013.514. J Invest Dermatol. 2014. PMID: 24732331 Free PMC article.
-
The Fraser Complex Proteins (Frem1, Frem2, and Fras1) Can Form Anchoring Cords in the Absence of AMACO at the Dermal-Epidermal Junction of Mouse Skin.Int J Mol Sci. 2023 Apr 5;24(7):6782. doi: 10.3390/ijms24076782. Int J Mol Sci. 2023. PMID: 37047755 Free PMC article.
-
Breakdown of the reciprocal stabilization of QBRICK/Frem1, Fras1, and Frem2 at the basement membrane provokes Fraser syndrome-like defects.Proc Natl Acad Sci U S A. 2006 Aug 8;103(32):11981-6. doi: 10.1073/pnas.0601011103. Epub 2006 Jul 31. Proc Natl Acad Sci U S A. 2006. PMID: 16880404 Free PMC article.
-
The role of Fras1/Frem proteins in the structure and function of basement membrane.Int J Biochem Cell Biol. 2011 Apr;43(4):487-95. doi: 10.1016/j.biocel.2010.12.016. Epub 2010 Dec 21. Int J Biochem Cell Biol. 2011. PMID: 21182980 Review.
-
Fraser syndrome: review of the literature illustrated by a historical adult case.Int J Oral Maxillofac Surg. 2020 Oct;49(10):1245-1253. doi: 10.1016/j.ijom.2020.01.007. Epub 2020 Jan 22. Int J Oral Maxillofac Surg. 2020. PMID: 31982235 Review.
Cited by
-
Cryptophthalmos, dental anomalies, oral vestibule defect, and a novel FREM2 mutation.J Hum Genet. 2022 Feb;67(2):115-118. doi: 10.1038/s10038-021-00972-4. Epub 2021 Aug 19. J Hum Genet. 2022. PMID: 34408272
-
Epigenetic and transcriptional dysregulation of VWA2 associated with a MYC-driven oncogenic program in colorectal cancer.Sci Rep. 2018 Jul 23;8(1):11097. doi: 10.1038/s41598-018-29378-7. Sci Rep. 2018. PMID: 30038405 Free PMC article.
-
A new component of the Fraser complex.J Invest Dermatol. 2014 May;134(5):1192-1193. doi: 10.1038/jid.2013.514. J Invest Dermatol. 2014. PMID: 24732331 Free PMC article.
-
Pharyngeal morphogenesis requires fras1-itga8-dependent epithelial-mesenchymal interaction.Dev Biol. 2016 Aug 1;416(1):136-148. doi: 10.1016/j.ydbio.2016.05.035. Epub 2016 Jun 2. Dev Biol. 2016. PMID: 27265864 Free PMC article.
-
The Fraser Complex Proteins (Frem1, Frem2, and Fras1) Can Form Anchoring Cords in the Absence of AMACO at the Dermal-Epidermal Junction of Mouse Skin.Int J Mol Sci. 2023 Apr 5;24(7):6782. doi: 10.3390/ijms24076782. Int J Mol Sci. 2023. PMID: 37047755 Free PMC article.
References
-
- Dalezios Y, Papasozomenos B, Petrou P, et al. Ultrastructural localization of Fras1 in the sublamina densa of embryonic epithelial basement membranes. Arch Dermatol Res. 2007;299:337–43. - PubMed
-
- Gebauer JM, Karlsen KR, Neiss WF, et al. Expression of the AMACO (VWA2 protein) ortholog in zebrafish. Gene Expr Patterns. 2010;10:53–59. - PubMed
-
- Gebauer JM, Keene DR, Olsen BR, et al. Mouse AMACO, a kidney and skin basement membrane associated molecule that mediates RGD-dependent cell attachment. Matrix Biol. 2009;28:456–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

