A double blind, within subject comparison of spontaneous opioid withdrawal from buprenorphine versus morphine
- PMID: 24227768
- PMCID: PMC3912547
- DOI: 10.1124/jpet.113.209478
A double blind, within subject comparison of spontaneous opioid withdrawal from buprenorphine versus morphine
Abstract
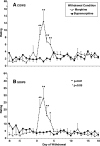
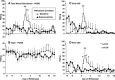
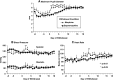
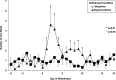
Preliminary evidence suggests that there is minimal withdrawal after the cessation of chronically administered buprenorphine and that opioid withdrawal symptoms are delayed compared with those of other opioids. The present study compared the time course and magnitude of buprenorphine withdrawal with a prototypical μ-opioid agonist, morphine. Healthy, out-of-treatment opioid-dependent residential volunteers (N = 7) were stabilized on either buprenorphine (32 mg/day i.m.) or morphine (120 mg/day i.m.) administered in four divided doses for 9 days. They then underwent an 18-day period of spontaneous withdrawal, during which four double-blind i.m. placebo injections were administered daily. Stabilization and spontaneous withdrawal were assessed for the second opioid using the same time course. Opioid withdrawal measures were collected eight times daily. Morphine withdrawal symptoms were significantly (P < 0.05) greater than those of buprenorphine withdrawal as measured by mean peak ratings of Clinical Opiate Withdrawal Scale (COWS), Subjective Opiate Withdrawal Scale (SOWS), all subscales of the Profile of Mood States (POMS), sick and pain (0-100) Visual Analog Scales, systolic and diastolic blood pressure, heart rate, respiratory rate, and pupil dilation. Peak ratings on COWS and SOWS occurred on day 2 of morphine withdrawal and were significantly greater than on day 2 of buprenorphine withdrawal. Subjective reports of morphine withdrawal resolved on average by day 7. There was minimal evidence of buprenorphine withdrawal on any measure. In conclusion, spontaneous withdrawal from high-dose buprenorphine appears subjectively and objectively milder compared with that of morphine for at least 18 days after drug cessation.
Figures
Similar articles
-
Efficacy of Tramadol Extended-Release for Opioid Withdrawal: A Randomized Clinical Trial.JAMA Psychiatry. 2017 Sep 1;74(9):885-893. doi: 10.1001/jamapsychiatry.2017.1838. JAMA Psychiatry. 2017. PMID: 28700791 Free PMC article. Clinical Trial.
-
Assessment of pioglitazone and proinflammatory cytokines during buprenorphine taper in patients with opioid use disorder.Psychopharmacology (Berl). 2018 Oct;235(10):2957-2966. doi: 10.1007/s00213-018-4986-5. Epub 2018 Aug 6. Psychopharmacology (Berl). 2018. PMID: 30079432 Free PMC article. Clinical Trial.
-
Extended-Release 7-Day Injectable Buprenorphine for Patients With Minimal to Mild Opioid Withdrawal.JAMA Netw Open. 2024 Jul 1;7(7):e2420702. doi: 10.1001/jamanetworkopen.2024.20702. JAMA Netw Open. 2024. PMID: 38976265 Free PMC article. Clinical Trial.
-
Medication-Assisted Treatment of Opioid Use Disorder in Adolescents and Young Adults.Adolesc Med State Art Rev. 2014 Aug;25(2):251-65. Adolesc Med State Art Rev. 2014. PMID: 27132312 Review. No abstract available.
-
Buprenorphine Treatment for Opioid Use Disorder: An Overview.CNS Drugs. 2019 Jun;33(6):567-580. doi: 10.1007/s40263-019-00637-z. CNS Drugs. 2019. PMID: 31062259 Free PMC article. Review.
Cited by
-
French-Canadian Translation and Cultural Adaptation of the Clinical Opiate Withdrawal Scale: The COWS-FC.Can J Psychiatry. 2022 Sep;67(9):701-711. doi: 10.1177/07067437221087066. Epub 2022 Mar 15. Can J Psychiatry. 2022. PMID: 35290134 Free PMC article.
-
Opiate withdrawal syndrome in buprenorphine abusers admitted to a rehabilitation center in Tunisia.Afr Health Sci. 2016 Dec;16(4):1067-1077. doi: 10.4314/ahs.v16i4.24. Afr Health Sci. 2016. PMID: 28479900 Free PMC article.
-
Buprenorphine for managing opioid withdrawal.Cochrane Database Syst Rev. 2017 Feb 21;2(2):CD002025. doi: 10.1002/14651858.CD002025.pub5. Cochrane Database Syst Rev. 2017. PMID: 28220474 Free PMC article. Review.
-
The Cholinergic System as a Treatment Target for Opioid Use Disorder.CNS Drugs. 2018 Nov;32(11):981-996. doi: 10.1007/s40263-018-0572-y. CNS Drugs. 2018. PMID: 30259415 Free PMC article. Review.
-
Buprenorphine for Chronic Pain: a Systemic Review.Curr Pain Headache Rep. 2018 Oct 5;22(12):83. doi: 10.1007/s11916-018-0732-2. Curr Pain Headache Rep. 2018. PMID: 30291571
References
-
- Amass L, Kamien JB, Mikulich SK. (2001) Thrice-weekly supervised dosing with the combination buprenorphine-naloxone tablet is preferred to daily supervised dosing by opioid-dependent humans. Drug Alcohol Depend 61:173–181 - PubMed
-
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders, American Psychiatric Association, Washington, D.C.
-
- Assadi SM, Hafezi M, Mokri A, Razzaghi EM, Ghaeli P. (2004) Opioid detoxification using high doses of buprenorphine in 24 hours: a randomized, double blind, controlled clinical trial. J Subst Abuse Treat 27:75–82 - PubMed
-
- Bastien CH, Vallières A, Morin CM. (2001) Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2:297–307 - PubMed
-
- Bloms-Funke P, Gillen C, Schuettler AJ, Wnendt S. (2000) Agonistic effects of the opioid buprenorphine on the nociceptin/OFQ receptor. Peptides 21:1141–1146 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
