Validation of the 12-gene colon cancer recurrence score in NSABP C-07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin
- PMID: 24220557
- PMCID: PMC3871512
- DOI: 10.1200/JCO.2012.47.3116
Validation of the 12-gene colon cancer recurrence score in NSABP C-07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin
Erratum in
- J Clin Oncol. 2014 Mar 10;32(8):866
Abstract
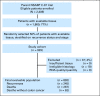
Purpose: Accurate assessments of recurrence risk and absolute treatment benefit are needed to inform colon cancer adjuvant therapy. The 12-gene Recurrence Score assay has been validated in patients with stage II colon cancer from the Cancer and Leukemia Group B 9581 and Quick and Simple and Reliable (QUASAR) trials. We conducted an independent, prospectively designed clinical validation study of Recurrence Score, with prespecified end points and analysis plan, in archival specimens from patients with stage II and III colon cancer randomly assigned to fluorouracil (FU) or FU plus oxaliplatin in National Surgical Adjuvant Breast and Bowel Project C-07.
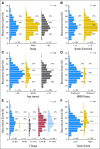
Methods: Recurrence Score was assessed in 892 fixed, paraffin-embedded tumor specimens (randomly selected 50% of patients with tissue). Data were analyzed by Cox regression adjusting for stage and treatment.
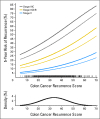
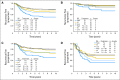
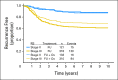
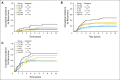
Results: Continuous Recurrence Score predicted recurrence (hazard ratio for a 25-unit increase in score, 1.96; 95% CI, 1.50 to 2.55; P < .001), as well as disease-free and overall survival (both P < .001). Recurrence Score predicted recurrence risk (P = .001) after adjustment for stage, mismatch repair, nodes examined, grade, and treatment. Recurrence Score did not have significant interaction with stage (P = .90) or age (P = .76). Relative benefit of oxaliplatin was similar across the range of Recurrence Score (interaction P = .48); accordingly, absolute benefit of oxaliplatin increased with higher scores, most notably in patients with stage II and IIIA/B disease.
Conclusion: The 12-gene Recurrence Score predicts recurrence risk in stage II and stage III colon cancer and provides additional information beyond conventional clinical and pathologic factors. Incorporating Recurrence Score into the clinical context may better inform adjuvant therapy decisions in stage III as well as stage II colon cancer.
Trial registration: ClinicalTrials.gov NCT00004931.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Clinical Outcome From Oxaliplatin Treatment in Stage II/III Colon Cancer According to Intrinsic Subtypes: Secondary Analysis of NSABP C-07/NRG Oncology Randomized Clinical Trial.JAMA Oncol. 2016 Sep 1;2(9):1162-9. doi: 10.1001/jamaoncol.2016.2314. JAMA Oncol. 2016. PMID: 27270348 Free PMC article. Clinical Trial.
-
Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study.J Clin Oncol. 2015 Dec 10;33(35):4176-87. doi: 10.1200/JCO.2015.63.4238. Epub 2015 Nov 2. J Clin Oncol. 2015. PMID: 26527776 Clinical Trial.
-
Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin.J Clin Oncol. 2010 Sep 1;28(25):3937-44. doi: 10.1200/JCO.2010.28.9538. Epub 2010 Aug 2. J Clin Oncol. 2010. PMID: 20679606 Free PMC article.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.Health Technol Assess. 2006 Nov;10(41):iii-iv, xi-xiv, 1-185. doi: 10.3310/hta10410. Health Technol Assess. 2006. PMID: 17049138 Review.
-
[Progression of adjuvant chemotherapy for colon cancer].Ai Zheng. 2005 Dec;24(12):1546-9. Ai Zheng. 2005. PMID: 16351811 Review. Chinese.
Cited by
-
Use of Molecular Assays and Circulating Tumor DNA in Early-Stage Colorectal Cancer: A Roundtable Discussion of the Gastrointestinal Cancer Therapy Expert Group.Oncologist. 2021 Aug;26(8):651-659. doi: 10.1002/onco.13738. Epub 2021 Mar 28. Oncologist. 2021. PMID: 33650740 Free PMC article.
-
Transcriptional subtyping and CD8 immunohistochemistry identifies poor prognosis stage II/III colorectal cancer patients who benefit from adjuvant chemotherapy.JCO Precis Oncol. 2018 Jun 13;2018:PO.17.00241. doi: 10.1200/PO.17.00241. JCO Precis Oncol. 2018. PMID: 30088816 Free PMC article.
-
Expressions of 10 genes as candidate predictors of recurrence in stage III colon cancer patients receiving adjuvant oxaliplatin-based chemotherapy.Oncol Lett. 2019 Aug;18(2):1388-1394. doi: 10.3892/ol.2019.10437. Epub 2019 Jun 5. Oncol Lett. 2019. PMID: 31423202 Free PMC article.
-
A stroma-related lncRNA panel for predicting recurrence and adjuvant chemotherapy benefit in patients with early-stage colon cancer.J Cell Mol Med. 2020 Mar;24(5):3229-3241. doi: 10.1111/jcmm.14999. Epub 2020 Jan 27. J Cell Mol Med. 2020. PMID: 31989761 Free PMC article.
-
Current status of gene expression profiling to assist decision making in stage II colon cancer.Oncologist. 2014 Jul;19(7):704-11. doi: 10.1634/theoncologist.2013-0471. Epub 2014 May 28. Oncologist. 2014. PMID: 24869929 Free PMC article.
References
-
- Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. - PubMed
-
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. - PubMed
-
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. - PubMed
-
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

