Evaluation of p21 promoter for interleukin 12 radiation induced transcriptional targeting in a mouse tumor model
- PMID: 24219565
- PMCID: PMC3832904
- DOI: 10.1186/1476-4598-12-136
Evaluation of p21 promoter for interleukin 12 radiation induced transcriptional targeting in a mouse tumor model
Abstract
Background: Radiation induced transcriptional targeting is a gene therapy approach that takes advantage of the targeting abilities of radiotherapy by using radio inducible promoters to spatially and temporally limit the transgene expression. Cyclin dependent kinase inhibitor 1 (CDKN1A), also known as p21, is a crucial regulator of the cell cycle, mediating G1 phase arrest in response to a variety of stress stimuli, including DNA damaging agents like irradiation. The aim of the study was to evaluate the suitability of the p21 promoter for radiation induced transcriptional targeting with the objective to test the therapeutic effectiveness of the combined radio-gene therapy with p21 promoter driven therapeutic gene interleukin 12.
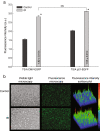
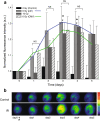
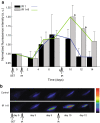
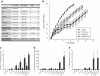
Methods: To test the inducibility of the p21 promoter, three reporter gene experimental models with green fluorescent protein (GFP) under the control of p21 promoter were established by gene electrotransfer of plasmid DNA: stably transfected cells, stably transfected tumors, and transiently transfected muscles. Induction of reporter gene expression after irradiation was determined using a fluorescence microplate reader in vitro and by non-invasive fluorescence imaging using fluorescence stereomicroscope in vivo. The antitumor effect of the plasmid encoding the p21 promoter driven interleukin 12 after radio-gene therapy was determined by tumor growth delay assay and by quantification of intratumoral and serum levels of interleukin 12 protein and intratumoral concentrations of interleukin 12 mRNA.
Results: Using the reporter gene experimental models, p21 promoter was proven to be inducible with radiation, the induction was not dose dependent, and it could be re-induced. Furthermore radio-gene therapy with interleukin 12 under control of the p21 promoter had a good antitumor therapeutic effect with the statistically relevant tumor growth delay, which was comparable to that of the same therapy using a constitutive promoter.
Conclusions: In this study p21 promoter was proven to be a suitable candidate for radiation induced transcriptional targeting. As a proof of principle the therapeutic value was demonstrated with the radio-inducible interleukin 12 plasmid providing a synergistic antitumor effect to radiotherapy alone, which makes this approach feasible for the combined treatment with radiotherapy.
Figures

Similar articles
-
Irradiation, cisplatin, and 5-azacytidine upregulate cytomegalovirus promoter in tumors and muscles: implementation of non-invasive fluorescence imaging.Mol Imaging Biol. 2011 Feb;13(1):43-52. doi: 10.1007/s11307-010-0300-6. Mol Imaging Biol. 2011. PMID: 20396957 Free PMC article.
-
P21-driven multifusion gene system for evaluating the efficacy of histone deacetylase inhibitors by in vivo molecular imaging and for transcription targeting therapy of cancer mediated by histone deacetylase inhibitor.J Nucl Med. 2014 Apr;55(4):678-85. doi: 10.2967/jnumed.113.126573. Epub 2014 Mar 17. J Nucl Med. 2014. PMID: 24639460
-
Quantitative analysis of p53-targeted gene expression and visualization of p53 transcriptional activity following intratumoral administration of adenoviral p53 in vivo.Mol Cancer Ther. 2004 Jan;3(1):93-100. Mol Cancer Ther. 2004. PMID: 14749479
-
Radiation therapy: activation for gene transcription and the development of genetic radiotherapy-therapeutic strategies in oncology.Cancer Biol Ther. 2003 Jul-Aug;2(4):326-9. doi: 10.4161/cbt.2.4.495. Cancer Biol Ther. 2003. PMID: 14508100 Review.
-
Radiogenic therapy: novel approaches for enhancing tumor radiosensitivity.Technol Cancer Res Treat. 2005 Aug;4(4):343-61. doi: 10.1177/153303460500400404. Technol Cancer Res Treat. 2005. PMID: 16029055 Review.
Cited by
-
Non-clinical evaluation of pmIL12 gene therapy for approval of the phase I clinical study.Sci Rep. 2024 Sep 27;14(1):22288. doi: 10.1038/s41598-024-73314-x. Sci Rep. 2024. PMID: 39333733 Free PMC article.
-
Tumor Radiosensitization by Gene Electrotransfer-Mediated Double Targeting of Tumor Vasculature.Int J Mol Sci. 2023 Feb 1;24(3):2755. doi: 10.3390/ijms24032755. Int J Mol Sci. 2023. PMID: 36769077 Free PMC article.
-
Non-Clinical In Vitro Evaluation of Antibiotic Resistance Gene-Free Plasmids Encoding Human or Murine IL-12 Intended for First-in-Human Clinical Study.Pharmaceutics. 2021 Oct 19;13(10):1739. doi: 10.3390/pharmaceutics13101739. Pharmaceutics. 2021. PMID: 34684032 Free PMC article.
-
Bioprofiling TS/A Murine Mammary Cancer for a Functional Precision Experimental Model.Cancers (Basel). 2019 Nov 27;11(12):1889. doi: 10.3390/cancers11121889. Cancers (Basel). 2019. PMID: 31783695 Free PMC article. Review.
-
Identification of potential genes and pathways for response prediction of neoadjuvant chemoradiotherapy in patients with rectal cancer by systemic biological analysis.Oncol Lett. 2019 Jan;17(1):492-501. doi: 10.3892/ol.2018.9598. Epub 2018 Oct 18. Oncol Lett. 2019. PMID: 30655792 Free PMC article.
References
-
- Bernier J, Hall EJ, Giaccia A. Timeline - radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4:737–U715. - PubMed
-
- Weichselbaum RR, Hallahan DE, Sukhatme VP, Kufe DW. Gene-therapy targeted by ionizing-radiation. Int J Radiat Oncol Biol Phys. 1992;24:565–567. - PubMed
-
- Kufe D, Weichselbaum R. Radiation therapy - activation of gene transcription and the development of genetic radiotherapy - therapeutic strategies in oncology. Cancer Biol Ther. 2003;2:326–329. - PubMed
-
- McCarthy O, Worthington J, Barrett E, Cosimo E, Boyd M, Mairs RJ, Ward C, McKeown SR, Hirst DG, Robson T. p(21(WAF1))-mediated transcriptional targeting of inducible nitric oxide synthase gene therapy sensitizes tumours to fractionated radiotherapy. Gene Ther. 2007;14:246–255. - PubMed
-
- Chastel C, Jiricny J, Jaussi R. Activation of stress-responsive promoters by ionizing radiation for deployment in targeted gene therapy. DNA Repair. 2004;3:683–684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

