Protein post-translational modifications and regulation of pluripotency in human stem cells
- PMID: 24217768
- PMCID: PMC3915910
- DOI: 10.1038/cr.2013.151
Protein post-translational modifications and regulation of pluripotency in human stem cells
Abstract
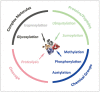
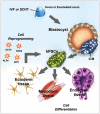
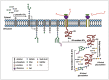
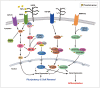
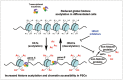
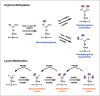
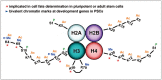
Post-translational modifications (PTMs) are known to be essential mechanisms used by eukaryotic cells to diversify their protein functions and dynamically coordinate their signaling networks. Defects in PTMs have been linked to numerous developmental disorders and human diseases, highlighting the importance of PTMs in maintaining normal cellular states. Human pluripotent stem cells (hPSCs), including embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), are capable of self-renewal and differentiation into a variety of functional somatic cells; these cells hold a great promise for the advancement of biomedical research and clinical therapy. The mechanisms underlying cellular pluripotency in human cells have been extensively explored in the past decade. In addition to the vast amount of knowledge obtained from the genetic and transcriptional research in hPSCs, there is a rapidly growing interest in the stem cell biology field to examine pluripotency at the protein and PTM level. This review addresses recent progress toward understanding the role of PTMs (glycosylation, phosphorylation, acetylation and methylation) in the regulation of cellular pluripotency.
Figures
Similar articles
-
Leucine-Rich Repeat Neuronal Protein 1 Regulates Differentiation of Embryonic Stem Cells by Post-Translational Modifications of Pluripotency Factors.Stem Cells. 2018 Oct;36(10):1514-1524. doi: 10.1002/stem.2862. Epub 2018 Jul 29. Stem Cells. 2018. PMID: 29893054
-
Ground rules of the pluripotency gene regulatory network.Nat Rev Genet. 2017 Mar;18(3):180-191. doi: 10.1038/nrg.2016.156. Epub 2017 Jan 3. Nat Rev Genet. 2017. PMID: 28045100 Review.
-
The 'sweet' spot of cellular pluripotency: protein glycosylation in human pluripotent stem cells and its applications in regenerative medicine.Expert Opin Biol Ther. 2015 May;15(5):679-87. doi: 10.1517/14712598.2015.1021329. Epub 2015 Mar 3. Expert Opin Biol Ther. 2015. PMID: 25736263 Review.
-
Molecular mechanisms involved in self-renewal and pluripotency of embryonic stem cells.J Cell Physiol. 2007 May;211(2):279-86. doi: 10.1002/jcp.20978. J Cell Physiol. 2007. PMID: 17195167 Review.
-
Oct4 maintains the pluripotency of human embryonic stem cells by inactivating p53 through Sirt1-mediated deacetylation.Stem Cells. 2014 Jan;32(1):157-65. doi: 10.1002/stem.1532. Stem Cells. 2014. PMID: 24038750 Free PMC article.
Cited by
-
Potential of histone deacetylase inhibitors in the control and regulation of prostate, breast and ovarian cancer.Front Chem. 2022 Aug 12;10:948217. doi: 10.3389/fchem.2022.948217. eCollection 2022. Front Chem. 2022. PMID: 36034650 Free PMC article. Review.
-
Virtual 2-D map of the fungal proteome.Sci Rep. 2021 Mar 23;11(1):6676. doi: 10.1038/s41598-021-86201-6. Sci Rep. 2021. PMID: 33758316 Free PMC article.
-
Cytosolic O-GlcNAcylation and PNG1 maintain Drosophila gut homeostasis by regulating proliferation and apoptosis.PLoS Genet. 2022 Mar 16;18(3):e1010128. doi: 10.1371/journal.pgen.1010128. eCollection 2022 Mar. PLoS Genet. 2022. PMID: 35294432 Free PMC article.
-
The impact of noise and missing fragmentation cleavages on de novo peptide identification algorithms.Comput Struct Biotechnol J. 2022 Mar 19;20:1402-1412. doi: 10.1016/j.csbj.2022.03.008. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35386104 Free PMC article.
-
Crystallographic mining of ASK1 regulators to unravel the intricate PPI interfaces for the discovery of small molecule.Comput Struct Biotechnol J. 2022 Jul 11;20:3734-3754. doi: 10.1016/j.csbj.2022.07.008. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35891784 Free PMC article. Review.
References
-
- Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. - PubMed
-
- Deribe YL, Pawson T, Dikic I. Post-translational modifications in signal integration. Nat Struct Mol Biol. 2010;17:666–672. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

