Reelin mobilizes a VAMP7-dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission
- PMID: 24210904
- PMCID: PMC3840105
- DOI: 10.1016/j.neuron.2013.08.024
Reelin mobilizes a VAMP7-dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission
Abstract
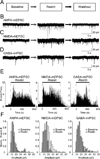
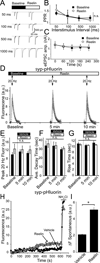
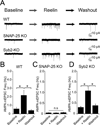
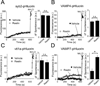
Reelin is a glycoprotein that is critical for proper layering of neocortex during development as well as dynamic regulation of glutamatergic postsynaptic signaling in mature synapses. Here, we show that Reelin also acts presynaptically, resulting in robust rapid enhancement of spontaneous neurotransmitter release without affecting properties of evoked neurotransmission. This effect of Reelin requires a modest but significant increase in presynaptic Ca(2+) initiated via ApoER2 signaling. The specificity of Reelin action on spontaneous neurotransmitter release is encoded at the level of vesicular SNARE machinery as it requires VAMP7 and SNAP-25 but not synaptobrevin2, VAMP4, or vti1a. These results uncover a presynaptic regulatory pathway that utilizes the heterogeneity of synaptic vesicle-associated SNAREs and selectively augments action potential-independent neurotransmission.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Role for Reelin in neurotransmitter release.J Neurosci. 2011 Feb 16;31(7):2352-60. doi: 10.1523/JNEUROSCI.3984-10.2011. J Neurosci. 2011. PMID: 21325502 Free PMC article.
-
Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission.Neuron. 2012 Jan 12;73(1):121-34. doi: 10.1016/j.neuron.2011.10.034. Neuron. 2012. PMID: 22243751 Free PMC article.
-
VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission.Nat Neurosci. 2012 Mar 11;15(5):738-45. doi: 10.1038/nn.3067. Nat Neurosci. 2012. PMID: 22406549 Free PMC article.
-
The role of reelin in adult synaptic function and the genetic and epigenetic regulation of the reelin gene.Biochim Biophys Acta. 2008 Aug;1779(8):422-31. doi: 10.1016/j.bbagrm.2008.01.001. Epub 2008 Jan 12. Biochim Biophys Acta. 2008. PMID: 18237558 Review.
-
Reelin glycoprotein in autism and schizophrenia.Int Rev Neurobiol. 2005;71:179-87. doi: 10.1016/s0074-7742(05)71008-4. Int Rev Neurobiol. 2005. PMID: 16512351 Review. No abstract available.
Cited by
-
Synaptic Vesicle Recycling and the Endolysosomal System: A Reappraisal of Form and Function.Front Synaptic Neurosci. 2022 Feb 25;14:826098. doi: 10.3389/fnsyn.2022.826098. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35280702 Free PMC article. Review.
-
Synaptic Vesicle Recycling Pathway Determines Neurotransmitter Content and Release Properties.Neuron. 2019 May 22;102(4):786-800.e5. doi: 10.1016/j.neuron.2019.03.031. Epub 2019 Apr 16. Neuron. 2019. PMID: 31003725 Free PMC article.
-
Spontaneous vesicle recycling in the synaptic bouton.Front Cell Neurosci. 2014 Dec 8;8:409. doi: 10.3389/fncel.2014.00409. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25538561 Free PMC article. Review.
-
Postnatal Role of the Cytoskeleton in Adult Epileptogenesis.Cereb Cortex Commun. 2020;1(1):tgaa024. doi: 10.1093/texcom/tgaa024. Epub 2020 Jun 17. Cereb Cortex Commun. 2020. PMID: 32864616 Free PMC article.
-
Selective Inactivation of Reelin in Inhibitory Interneurons Leads to Subtle Changes in the Dentate Gyrus But Leaves Cortical Layering and Behavior Unaffected.Cereb Cortex. 2020 Mar 14;30(3):1688-1707. doi: 10.1093/cercor/bhz196. Cereb Cortex. 2020. PMID: 31667489 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

