Cooperative functions of ZnT1, metallothionein and ZnT4 in the cytoplasm are required for full activation of TNAP in the early secretory pathway
- PMID: 24204829
- PMCID: PMC3799634
- DOI: 10.1371/journal.pone.0077445
Cooperative functions of ZnT1, metallothionein and ZnT4 in the cytoplasm are required for full activation of TNAP in the early secretory pathway
Abstract
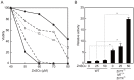
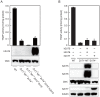
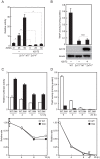
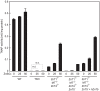
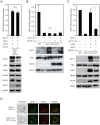
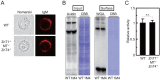
The activation process of secretory or membrane-bound zinc enzymes is thought to be a highly coordinated process involving zinc transport, trafficking, transfer and coordination. We have previously shown that secretory and membrane-bound zinc enzymes are activated in the early secretory pathway (ESP) via zinc-loading by the zinc transporter 5 (ZnT5)-ZnT6 hetero-complex and ZnT7 homo-complex (zinc transport complexes). However, how other proteins conducting zinc metabolism affect the activation of these enzymes remains unknown. Here, we investigated this issue by disruption and re-expression of genes known to be involved in cytoplasmic zinc metabolism, using a zinc enzyme, tissue non-specific alkaline phosphatase (TNAP), as a reporter. We found that TNAP activity was significantly reduced in cells deficient in ZnT1, Metallothionein (MT) and ZnT4 genes (ZnT1(-/-) MT(-/-) ZnT4(-/-) cells), in spite of increased cytosolic zinc levels. The reduced TNAP activity in ZnT1(-/-) MT(-/-) ZnT4(-/-) cells was not restored when cytosolic zinc levels were normalized to levels comparable with those of wild-type cells, but was reversely restored by extreme zinc supplementation via zinc-loading by the zinc transport complexes. Moreover, the reduced TNAP activity was adequately restored by re-expression of mammalian counterparts of ZnT1, MT and ZnT4, but not by zinc transport-incompetent mutants of ZnT1 and ZnT4. In ZnT1(-/-) MT(-/-) ZnT4(-/-) cells, the secretory pathway normally operates. These findings suggest that cooperative zinc handling of ZnT1, MT and ZnT4 in the cytoplasm is required for full activation of TNAP in the ESP, and present clear evidence that the activation process of zinc enzymes is elaborately controlled.
Conflict of interest statement
Figures

Similar articles
-
Tissue nonspecific alkaline phosphatase is activated via a two-step mechanism by zinc transport complexes in the early secretory pathway.J Biol Chem. 2011 May 6;286(18):16363-73. doi: 10.1074/jbc.M111.227173. Epub 2011 Mar 14. J Biol Chem. 2011. PMID: 21402707 Free PMC article.
-
Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells.J Biol Chem. 2005 Sep 2;280(35):30956-62. doi: 10.1074/jbc.M506902200. Epub 2005 Jul 1. J Biol Chem. 2005. PMID: 15994300
-
Dissecting the Process of Activation of Cancer-promoting Zinc-requiring Ectoenzymes by Zinc Metalation Mediated by ZNT Transporters.J Biol Chem. 2017 Feb 10;292(6):2159-2173. doi: 10.1074/jbc.M116.763946. Epub 2016 Dec 27. J Biol Chem. 2017. PMID: 28028180 Free PMC article.
-
Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers.Pflugers Arch. 2004 Feb;447(5):744-51. doi: 10.1007/s00424-003-1070-7. Epub 2003 May 14. Pflugers Arch. 2004. PMID: 12748859 Review.
-
Metallothionein and Zinc Transporter Expression in Circulating Human Blood Cells as Biomarkers of Zinc Status: a Systematic Review.Adv Nutr. 2016 Jul 15;7(4):735-46. doi: 10.3945/an.116.012518. Print 2016 Jul. Adv Nutr. 2016. PMID: 27422508 Free PMC article. Review.
Cited by
-
The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective.Int J Mol Sci. 2016 Mar 4;17(3):336. doi: 10.3390/ijms17030336. Int J Mol Sci. 2016. PMID: 26959009 Free PMC article. Review.
-
Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis.J Physiol Sci. 2017 Mar;67(2):283-301. doi: 10.1007/s12576-017-0521-4. Epub 2017 Jan 27. J Physiol Sci. 2017. PMID: 28130681 Free PMC article. Review.
-
Loss-of-function SLC30A2 mutants are associated with gut dysbiosis and alterations in intestinal gene expression in preterm infants.Gut Microbes. 2022 Jan-Dec;14(1):2014739. doi: 10.1080/19490976.2021.2014739. Gut Microbes. 2022. PMID: 34965180 Free PMC article.
-
The PP-motif in luminal loop 2 of ZnT transporters plays a pivotal role in TNAP activation.Biochem J. 2016 Sep 1;473(17):2611-21. doi: 10.1042/BCJ20160324. Epub 2016 Jun 14. Biochem J. 2016. PMID: 27303047 Free PMC article.
-
Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels.J Biol Chem. 2019 Oct 25;294(43):15686-15697. doi: 10.1074/jbc.RA119.010227. Epub 2019 Aug 30. J Biol Chem. 2019. PMID: 31471319 Free PMC article.
References
-
- Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73: 79–118. - PubMed
-
- Maret W (2012) New perspectives of zinc coordination environments in proteins. J Inorg Biochem 111: 110–116. - PubMed
-
- Andreini C, Banci L, Bertini I, Rosato A (2006) Counting the zinc-proteins encoded in the human genome. J Proteome Res 5: 196–201. - PubMed
-
- Maret W, Li Y (2009) Coordination dynamics of zinc in proteins. Chem Rev 109: 4682–4707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

