IRF-4-mediated CIITA transcription is blocked by KSHV encoded LANA to inhibit MHC II presentation
- PMID: 24204280
- PMCID: PMC3814934
- DOI: 10.1371/journal.ppat.1003751
IRF-4-mediated CIITA transcription is blocked by KSHV encoded LANA to inhibit MHC II presentation
Abstract
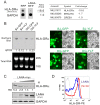
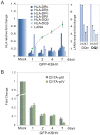
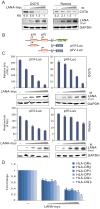
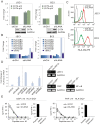
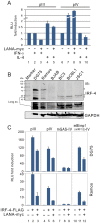
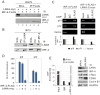
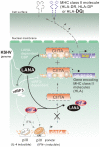
Peptides presentation to T cells by MHC class II molecules is of importance in initiation of immune response to a pathogen. The level of MHC II expression directly influences T lymphocyte activation and is often targeted by various viruses. Kaposi's sarcoma-associated herpesvirus (KSHV) encoded LANA is known to evade MHC class I peptide processing, however, the effect of LANA on MHC class II remains unclear. Here, we report that LANA down-regulates MHC II expression and presentation by inhibiting the transcription of MHC II transactivator (CIITA) promoter pIII and pIV in a dose-dependent manner. Strikingly, although LANA knockdown efficiently disrupts the inhibition of CIITA transcripts from its pIII and pIV promoter region, the expression of HLA-DQβ but no other MHC II molecules was significantly restored. Moreover, we revealed that the presentation of HLA-DQβ enhanced by LANA knockdown did not help LANA-specific CD4+ T cell recognition of PEL cells, and the inhibition of CIITA by LANA is independent of IL-4 or IFN-γ signaling but dependent on the direct interaction of LANA with IRF-4 (an activator of both the pIII and pIV CIITA promoters). This interaction dramatically blocked the DNA-binding ability of IRF-4 on both pIII and pIV promoters. Thus, our data implies that LANA can evade MHC II presentation and suppress CIITA transcription to provide a unique strategy of KSHV escape from immune surveillance by cytotoxic T cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen Inhibits Major Histocompatibility Complex Class II Expression by Disrupting Enhanceosome Assembly through Binding with the Regulatory Factor X Complex.J Virol. 2015 May;89(10):5536-56. doi: 10.1128/JVI.03713-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740990 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 3 inhibits gamma interferon and major histocompatibility complex class II expression.J Virol. 2011 May;85(9):4530-7. doi: 10.1128/JVI.02123-10. Epub 2011 Feb 23. J Virol. 2011. PMID: 21345951 Free PMC article.
-
Malignant glioma cells use MHC class II transactivator (CIITA) promoters III and IV to direct IFN-gamma-inducible CIITA expression and can function as nonprofessional antigen presenting cells in endocytic processing and CD4(+) T-cell activation.Glia. 2001 Dec;36(3):391-405. doi: 10.1002/glia.1125. Glia. 2001. PMID: 11746775
-
Kaposi's sarcoma-associated herpesvirus-encoded viral IRF3 modulates major histocompatibility complex class II (MHC-II) antigen presentation through MHC-II transactivator-dependent and -independent mechanisms: implications for oncogenesis.J Virol. 2013 May;87(10):5340-50. doi: 10.1128/JVI.00250-13. Epub 2013 Feb 28. J Virol. 2013. PMID: 23449805 Free PMC article.
-
Activated Nrf2 Interacts with Kaposi's Sarcoma-Associated Herpesvirus Latency Protein LANA-1 and Host Protein KAP1 To Mediate Global Lytic Gene Repression.J Virol. 2015 Aug;89(15):7874-92. doi: 10.1128/JVI.00895-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995248 Free PMC article.
Cited by
-
Correspondence on editorial regarding "Novel role of MHC class II transactivator in hepatitis B virus replication and viral counteraction".Clin Mol Hepatol. 2024 Oct;30(4):1028-1030. doi: 10.3350/cmh.2024.0515. Epub 2024 Jul 9. Clin Mol Hepatol. 2024. PMID: 38978449 Free PMC article. No abstract available.
-
Major Histocompatibility Complex Class II HLA-DRα Is Downregulated by Kaposi's Sarcoma-Associated Herpesvirus-Encoded Lytic Transactivator RTA and MARCH8.J Virol. 2016 Aug 26;90(18):8047-58. doi: 10.1128/JVI.01079-16. Print 2016 Sep 15. J Virol. 2016. PMID: 27356905 Free PMC article.
-
Ebola virus secreted glycoprotein decreases the anti-viral immunity of macrophages in early inflammatory responses.Cell Immunol. 2018 Feb;324:24-32. doi: 10.1016/j.cellimm.2017.11.009. Epub 2017 Nov 27. Cell Immunol. 2018. PMID: 29195741 Free PMC article.
-
Class II transactivator restricts viral replication, extending its effect to HBV: Editorial on "Novel role of MHC class II transactivator in hepatitis B virus replication and viral counteraction".Clin Mol Hepatol. 2024 Oct;30(4):724-727. doi: 10.3350/cmh.2024.0465. Epub 2024 Jul 3. Clin Mol Hepatol. 2024. PMID: 38957141 Free PMC article. No abstract available.
-
Impact of HVT Vaccination on Splenic miRNA Expression in Marek's Disease Virus Infections.Genes (Basel). 2019 Feb 5;10(2):115. doi: 10.3390/genes10020115. Genes (Basel). 2019. PMID: 30764490 Free PMC article.
References
-
- Matechak EO, Killeen N, Hedrick SM, Fowlkes BJ (1996) MHC class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity 4: 337–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

