Nuclear interferon-inducible protein 16 promotes silencing of herpesviral and transfected DNA
- PMID: 24198334
- PMCID: PMC3839728
- DOI: 10.1073/pnas.1316194110
Nuclear interferon-inducible protein 16 promotes silencing of herpesviral and transfected DNA
Abstract
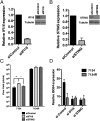
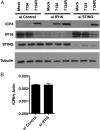
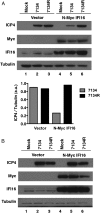
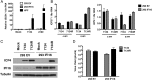
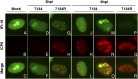
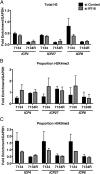
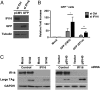
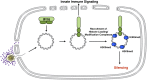
Mammalian cells have evolved mechanisms to silence foreign DNA introduced by viruses or by transfection. Upon herpesviral infection of cells, the viral genome is chromatinized in an attempt by the host cell to restrict expression of the viral genome. HSV ICP0 acts to counter host-intrinsic and innate responses to viral infection. We have found that nuclear interferon (IFN)-inducible protein 16 (IFI16) acts as a restriction factor against ICP0-null herpes simplex virus 1 (HSV-1) to limit viral replication and immediate-early gene expression. IFI16 promoted the addition of heterochromatin marks and the reduction of euchromatin marks on viral chromatin. IFI16 also restricted the expression of plasmid DNAs introduced by transfection but did not restrict SV40 DNA introduced into the cellular nucleus in the form of nucleosomal chromatin by viral infection. These results argue that IFI16 restricts unchromatinized DNA when it enters the cell nucleus by promoting the loading of nucleosomes and the addition of heterochromatin marks. Furthermore, these results indicate that IFI16 provides a broad surveillance role against viral and transfected DNA by promoting restriction of gene expression from the exogenous DNA and inducing innate immune responses.
Keywords: DNA sensing; cellular response; host restriction; intrinsic resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Nuclear DNA Sensor IFI16 Acts as a Restriction Factor for Human Papillomavirus Replication through Epigenetic Modifications of the Viral Promoters.J Virol. 2015 Aug;89(15):7506-20. doi: 10.1128/JVI.00013-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972554 Free PMC article.
-
Mechanisms of Host IFI16, PML, and Daxx Protein Restriction of Herpes Simplex Virus 1 Replication.J Virol. 2018 Apr 27;92(10):e00057-18. doi: 10.1128/JVI.00057-18. Print 2018 May 15. J Virol. 2018. PMID: 29491153 Free PMC article.
-
Role for a Filamentous Nuclear Assembly of IFI16, DNA, and Host Factors in Restriction of Herpesviral Infection.mBio. 2019 Jan 22;10(1):e02621-18. doi: 10.1128/mBio.02621-18. mBio. 2019. PMID: 30670617 Free PMC article.
-
Nuclear sensing of viral DNA, epigenetic regulation of herpes simplex virus infection, and innate immunity.Virology. 2015 May;479-480:153-9. doi: 10.1016/j.virol.2015.02.009. Epub 2015 Mar 3. Virology. 2015. PMID: 25742715 Free PMC article. Review.
-
The interferon-inducible DNA-sensor protein IFI16: a key player in the antiviral response.New Microbiol. 2015 Jan;38(1):5-20. Epub 2015 Jan 1. New Microbiol. 2015. PMID: 25742143 Review.
Cited by
-
Inflammasome, the Constitutive Heterochromatin Machinery, and Replication of an Oncogenic Herpesvirus.Viruses. 2021 May 6;13(5):846. doi: 10.3390/v13050846. Viruses. 2021. PMID: 34066537 Free PMC article. Review.
-
Infected cell protein 0 functional domains and their coordination in herpes simplex virus replication.World J Virol. 2016 Feb 12;5(1):1-13. doi: 10.5501/wjv.v5.i1.1. World J Virol. 2016. PMID: 26870669 Free PMC article. Review.
-
cGAS-STING-TBK1 Signaling Promotes Valproic Acid-Responsive Human Cytomegalovirus Immediate-Early Transcription during Infection of Incompletely Differentiated Myeloid Cells.Viruses. 2024 May 30;16(6):877. doi: 10.3390/v16060877. Viruses. 2024. PMID: 38932169 Free PMC article.
-
Single-genome analysis reveals a heterogeneous association of the herpes simplex virus genome with H3K27me2 and the reader PHF20L1 following infection of human fibroblasts.mBio. 2024 Apr 10;15(4):e0327823. doi: 10.1128/mbio.03278-23. Epub 2024 Feb 27. mBio. 2024. PMID: 38411116 Free PMC article.
-
A novel transcript isoform of STING that sequesters cGAMP and dominantly inhibits innate nucleic acid sensing.Nucleic Acids Res. 2018 May 4;46(8):4054-4071. doi: 10.1093/nar/gky186. Nucleic Acids Res. 2018. PMID: 29547894 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

