Quality control of Photosystem II: reversible and irreversible protein aggregation decides the fate of Photosystem II under excessive illumination
- PMID: 24194743
- PMCID: PMC3810940
- DOI: 10.3389/fpls.2013.00433
Quality control of Photosystem II: reversible and irreversible protein aggregation decides the fate of Photosystem II under excessive illumination
Abstract
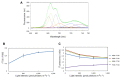
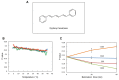
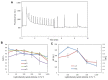
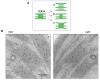
In response to excessive light, the thylakoid membranes of higher plant chloroplasts show dynamic changes including the degradation and reassembly of proteins, a change in the distribution of proteins, and large-scale structural changes such as unstacking of the grana. Here, we examined the aggregation of light-harvesting chlorophyll-protein complexes and Photosystem II core subunits of spinach thylakoid membranes under light stress with 77K chlorophyll fluorescence; aggregation of these proteins was found to proceed with increasing light intensity. Measurement of changes in the fluidity of thylakoid membranes with fluorescence polarization of diphenylhexatriene showed that membrane fluidity increased at a light intensity of 500-1,000 μmol photons m(-) (2) s(-) (1), and decreased at very high light intensity (1,500 μmol photons m(-) (2) s(-) (1)). The aggregation of light-harvesting complexes at moderately high light intensity is known to be reversible, while that of Photosystem II core subunits at extremely high light intensity is irreversible. It is likely that the reversibility of protein aggregation is closely related to membrane fluidity: increases in fluidity should stimulate reversible protein aggregation, whereas irreversible protein aggregation might decrease membrane fluidity. When spinach leaves were pre-illuminated with moderately high light intensity, the qE component of non-photochemical quenching and the optimum quantum yield of Photosystem II increased, indicating that Photosystem II/light-harvesting complexes rearranged in the thylakoid membranes to optimize Photosystem II activity. Transmission electron microscopy revealed that the thylakoids underwent partial unstacking under these light stress conditions. Thus, protein aggregation is involved in thylakoid dynamics and regulates photochemical reactions, thereby deciding the fate of Photosystem II.
Keywords: Photosystem II; lipid peroxidation; membrane dynamics; non-photochemical quenching; photoinhibition; protein aggregation; quality control mechanism; thylakoid unstacking.
Figures
Similar articles
-
Quality Control of Photosystem II: The Mechanisms for Avoidance and Tolerance of Light and Heat Stresses are Closely Linked to Membrane Fluidity of the Thylakoids.Front Plant Sci. 2016 Aug 2;7:1136. doi: 10.3389/fpls.2016.01136. eCollection 2016. Front Plant Sci. 2016. PMID: 27532009 Free PMC article. Review.
-
Quality control of PSII: behavior of PSII in the highly crowded grana thylakoids under excessive light.Plant Cell Physiol. 2014 Jul;55(7):1206-15. doi: 10.1093/pcp/pcu043. Epub 2014 Mar 7. Plant Cell Physiol. 2014. PMID: 24610582 Free PMC article. Review.
-
Quality control of photosystem II: lipid peroxidation accelerates photoinhibition under excessive illumination.PLoS One. 2012;7(12):e52100. doi: 10.1371/journal.pone.0052100. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300595 Free PMC article.
-
Quality control of Photosystem II: the molecular basis for the action of FtsH protease and the dynamics of the thylakoid membranes.J Photochem Photobiol B. 2014 Aug;137:100-6. doi: 10.1016/j.jphotobiol.2014.02.012. Epub 2014 Mar 4. J Photochem Photobiol B. 2014. PMID: 24725639 Review.
-
Quality control of photosystem II: direct imaging of the changes in the thylakoid structure and distribution of FtsH proteases in spinach chloroplasts under light stress.Plant Cell Physiol. 2014 Jul;55(7):1255-65. doi: 10.1093/pcp/pcu079. Epub 2014 Jun 1. Plant Cell Physiol. 2014. PMID: 24891560
Cited by
-
Changes in Photosystem II Complex and Physiological Activities in Pea and Maize Plants in Response to Salt Stress.Plants (Basel). 2024 Apr 3;13(7):1025. doi: 10.3390/plants13071025. Plants (Basel). 2024. PMID: 38611554 Free PMC article.
-
Small-Angle X-Ray and Neutron Scattering on Photosynthetic Membranes.Front Chem. 2021 Apr 19;9:631370. doi: 10.3389/fchem.2021.631370. eCollection 2021. Front Chem. 2021. PMID: 33954157 Free PMC article. Review.
-
The pattern of photosynthetic response and adaptation to changing light conditions in lichens is linked to their ecological range.Photosynth Res. 2023 Jul;157(1):21-35. doi: 10.1007/s11120-023-01015-z. Epub 2023 Mar 28. Photosynth Res. 2023. PMID: 36976446 Free PMC article.
-
Born in 1949 in postwar Japan.Photosynth Res. 2016 Jan;127(1):25-32. doi: 10.1007/s11120-014-0072-y. Epub 2015 Jan 4. Photosynth Res. 2016. PMID: 25557391
-
Induction events and short-term regulation of electron transport in chloroplasts: an overview.Photosynth Res. 2015 Aug;125(1-2):65-94. doi: 10.1007/s11120-015-0094-0. Epub 2015 Feb 14. Photosynth Res. 2015. PMID: 25680580 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

