Nitric oxide in guard cells as an important secondary messenger during stomatal closure
- PMID: 24194741
- PMCID: PMC3810675
- DOI: 10.3389/fpls.2013.00425
Nitric oxide in guard cells as an important secondary messenger during stomatal closure
Abstract
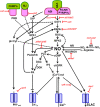
The modulation of guard cell function is the basis of stomatal closure, essential for optimizing water use and CO2 uptake by leaves. Nitric oxide (NO) in guard cells plays a very important role as a secondary messenger during stomatal closure induced by effectors, including hormones. For example, exposure to abscisic acid (ABA) triggers a marked increase in NO of guard cells, well before stomatal closure. In guard cells of multiple species, like Arabidopsis, Vicia and pea, exposure to ABA or methyl jasmonate or even microbial elicitors (e.g., chitosan) induces production of NO as well as reactive oxygen species (ROS). The role of NO in stomatal closure has been confirmed by using NO donors (e.g., SNP) and NO scavengers (like cPTIO) and inhibitors of NOS (L-NAME) or NR (tungstate). Two enzymes: a L-NAME-sensitive, nitric oxide synthase (NOS)-like enzyme and a tungstate-sensitive nitrate reductase (NR), can mediate ABA-induced NO rise in guard cells. However, the existence of true NOS in plant tissues and its role in guard cell NO-production are still a matter of intense debate. Guard cell signal transduction leading to stomatal closure involves the participation of several components, besides NO, such as cytosolic pH, ROS, free Ca(2+), and phospholipids. Use of fluorescent dyes has revealed that the rise in NO of guard cells occurs after the increase in cytoplasmic pH and ROS. The rise in NO causes an elevation in cytosolic free Ca(2+) and promotes the efflux of cations as well as anions from guard cells. Stomatal guard cells have become a model system to study the signaling cascade mechanisms in plants, particularly with NO as a dominant component. The interrelationships and interactions of NO with cytosolic pH, ROS, and free Ca(2+) are quite complex and need further detailed examination. While assessing critically the available literature, the present review projects possible areas of further work related to NO-action in stomatal guard cells.
Keywords: abscisic acid; cytosolic pH; elicitors; phospholipids; polyamines; reactive oxygen species; signal transduction.
Figures
Similar articles
-
Convergence and Divergence of Signaling Events in Guard Cells during Stomatal Closure by Plant Hormones or Microbial Elicitors.Front Plant Sci. 2016 Aug 24;7:1332. doi: 10.3389/fpls.2016.01332. eCollection 2016. Front Plant Sci. 2016. PMID: 27605934 Free PMC article. Review.
-
Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress.Adv Exp Med Biol. 2018;1081:215-232. doi: 10.1007/978-981-13-1244-1_12. Adv Exp Med Biol. 2018. PMID: 30288712 Review.
-
Nitric oxide production occurs downstream of reactive oxygen species in guard cells during stomatal closure induced by chitosan in abaxial epidermis of Pisum sativum.Planta. 2009 Mar;229(4):757-65. doi: 10.1007/s00425-008-0855-5. Epub 2008 Dec 16. Planta. 2009. PMID: 19084995
-
Stomatal Closure and Rise in ROS/NO of Arabidopsis Guard Cells by Tobacco Microbial Elicitors: Cryptogein and Harpin.Front Plant Sci. 2017 Jun 21;8:1096. doi: 10.3389/fpls.2017.01096. eCollection 2017. Front Plant Sci. 2017. PMID: 28680439 Free PMC article.
-
Nitric oxide production occurs after cytosolic alkalinization during stomatal closure induced by abscisic acid.Plant Cell Environ. 2008 Nov;31(11):1717-24. doi: 10.1111/j.1365-3040.2008.01872.x. Epub 2008 Sep 16. Plant Cell Environ. 2008. PMID: 18721267
Cited by
-
PLC1 mediated Cycloastragenol-induced stomatal movement by regulating the production of NO in Arabidopsis thaliana.BMC Plant Biol. 2023 Nov 17;23(1):571. doi: 10.1186/s12870-023-04555-7. BMC Plant Biol. 2023. PMID: 37978426 Free PMC article.
-
Multiple cyclic nucleotide-gated channels function as ABA-activated Ca2+ channels required for ABA-induced stomatal closure in Arabidopsis.Plant Cell. 2023 Jan 2;35(1):239-259. doi: 10.1093/plcell/koac274. Plant Cell. 2023. PMID: 36069643 Free PMC article.
-
Update on roles of nitric oxide in regulating stomatal closure.Plant Signal Behav. 2019;14(10):e1649569. doi: 10.1080/15592324.2019.1649569. Epub 2019 Aug 1. Plant Signal Behav. 2019. PMID: 31370725 Free PMC article. Review.
-
Stomatal closure induced by phytosphingosine-1-phosphate and sphingosine-1-phosphate depends on nitric oxide and pH of guard cells in Pisum sativum.Planta. 2016 Oct;244(4):831-41. doi: 10.1007/s00425-016-2545-z. Epub 2016 May 27. Planta. 2016. PMID: 27233507
-
A network-based modeling framework reveals the core signal transduction network underlying high carbon dioxide-induced stomatal closure in guard cells.PLoS Biol. 2024 May 1;22(5):e3002592. doi: 10.1371/journal.pbio.3002592. eCollection 2024 May. PLoS Biol. 2024. PMID: 38691548 Free PMC article.
References
-
- Alcázar R., Cuevas J. C., Patron M., Altabella T., Tiburcio A. F. (2006). Abscisic acid modulates polyamine metabolism under water stress in Arabidopsis thaliana. Physiol. Plant. 128, 448–455 10.1111/j.1399-3054.2006.00780.x - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

