Binding to E1 and E3 is mutually exclusive for the human autophagy E2 Atg3
- PMID: 24186333
- PMCID: PMC3843624
- DOI: 10.1002/pro.2381
Binding to E1 and E3 is mutually exclusive for the human autophagy E2 Atg3
Abstract
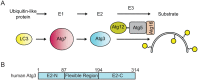
Ubiquitin-like proteins (UBLs) are activated, transferred and conjugated by E1-E2-E3 enzyme cascades. E2 enzymes for canonical UBLs such as ubiquitin, SUMO, and NEDD8 typically use common surfaces to bind to E1 and E3 enzymes. Thus, canonical E2s are required to disengage from E1 prior to E3-mediated UBL ligation. However, E1, E2, and E3 enzymes in the autophagy pathway are structurally and functionally distinct from canonical enzymes, and it has not been possible to predict whether autophagy UBL cascades are organized according to the same principles. Here, we address this question for the pathway mediating lipidation of the human autophagy UBL, LC3. We utilized bioinformatic and experimental approaches to identify a distinctive region in the autophagy E2, Atg3, that binds to the autophagy E3, Atg12∼Atg5-Atg16. Short peptides corresponding to this Atg3 sequence inhibit LC3 lipidation in vitro. Notably, the E3-binding site on Atg3 overlaps with the binding site for the E1, Atg7. Accordingly, the E3 competes with Atg7 for binding to Atg3, implying that Atg3 likely cycles back and forth between binding to Atg7 for loading with the UBL LC3 and binding to E3 to promote LC3 lipidation. The results show that common organizational principles underlie canonical and noncanonical UBL transfer cascades, but are established through distinct structural features.
Keywords: Atg12∼Atg5; Atg3; Atg7; E1 enzyme; E1‐E2 binding; E2 enzyme; E2‐E3 binding; E3 enzyme; autophagy; ubiquitin‐like protein.
© 2013 The Protein Society.
Figures


Similar articles
-
A switch element in the autophagy E2 Atg3 mediates allosteric regulation across the lipidation cascade.Nat Commun. 2019 Aug 9;10(1):3600. doi: 10.1038/s41467-019-11435-y. Nat Commun. 2019. PMID: 31399562 Free PMC article.
-
Allosteric regulation through a switch element in the autophagy E2, Atg3.Autophagy. 2020 Jan;16(1):183-184. doi: 10.1080/15548627.2019.1688550. Epub 2019 Nov 9. Autophagy. 2020. PMID: 31690182 Free PMC article. Review.
-
Structures of Atg7-Atg3 and Atg7-Atg10 reveal noncanonical mechanisms of E2 recruitment by the autophagy E1.Autophagy. 2013 May;9(5):778-80. doi: 10.4161/auto.23644. Epub 2013 Feb 6. Autophagy. 2013. PMID: 23388412 Free PMC article.
-
Noncanonical E2 recruitment by the autophagy E1 revealed by Atg7-Atg3 and Atg7-Atg10 structures.Nat Struct Mol Biol. 2012 Dec;19(12):1242-9. doi: 10.1038/nsmb.2415. Epub 2012 Nov 11. Nat Struct Mol Biol. 2012. PMID: 23142976 Free PMC article.
-
Structural insights into E2-E3 interaction for LC3 lipidation.Autophagy. 2014 Mar;10(3):522-3. doi: 10.4161/auto.27594. Epub 2014 Jan 9. Autophagy. 2014. PMID: 24413923 Free PMC article. Review.
Cited by
-
Structural Analyses of a GABARAP~ATG3 Conjugate Uncover a Novel Non-covalent Ubl-E2 Backside Interaction.bioRxiv [Preprint]. 2024 Aug 15:2024.08.14.607425. doi: 10.1101/2024.08.14.607425. bioRxiv. 2024. PMID: 39185234 Free PMC article. Preprint.
-
Identification and characterization of the linear region of ATG3 that interacts with ATG7 in higher eukaryotes.Biochem Biophys Res Commun. 2015 Jul 31;463(3):447-52. doi: 10.1016/j.bbrc.2015.05.107. Epub 2015 Jun 2. Biochem Biophys Res Commun. 2015. PMID: 26043688 Free PMC article.
-
Ubiquitin-like Protein Conjugation: Structures, Chemistry, and Mechanism.Chem Rev. 2018 Feb 14;118(3):889-918. doi: 10.1021/acs.chemrev.6b00737. Epub 2017 Feb 24. Chem Rev. 2018. PMID: 28234446 Free PMC article. Review.
-
Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay.Elife. 2016 Jan 26;5:e12245. doi: 10.7554/eLife.12245. Elife. 2016. PMID: 26812546 Free PMC article.
-
Post-translationally-modified structures in the autophagy machinery: an integrative perspective.FEBS J. 2015 Sep;282(18):3474-88. doi: 10.1111/febs.13356. Epub 2015 Jul 16. FEBS J. 2015. PMID: 26108642 Free PMC article. Review.
References
-
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. - PubMed
-
- Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. - PubMed
-
- Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

