A molecular mechanism regulating the timing of corticogeniculate innervation
- PMID: 24183669
- PMCID: PMC3849812
- DOI: 10.1016/j.celrep.2013.09.041
A molecular mechanism regulating the timing of corticogeniculate innervation
Abstract
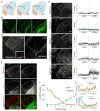
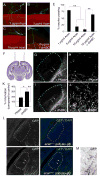
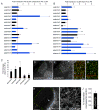
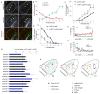
Neural circuit formation demands precise timing of innervation by different classes of axons. However, the mechanisms underlying such activity remain largely unknown. In the dorsal lateral geniculate nucleus (dLGN), axons from the retina and visual cortex innervate thalamic relay neurons in a highly coordinated manner, with those from the cortex arriving well after those from retina. The differential timing of retino- and corticogeniculate innervation is not a coincidence but is orchestrated by retinal inputs. Here, we identified a chondroitin sulfate proteoglycan (CSPG) that regulates the timing of corticogeniculate innervation. Aggrecan, a repulsive CSPG, is enriched in neonatal dLGN and inhibits cortical axons from prematurely entering the dLGN. Postnatal loss of aggrecan from dLGN coincides with upregulation of aggrecanase expression in the dLGN and corticogeniculate innervation and, it is important to note, is regulated by retinal inputs. Taken together, these studies reveal a molecular mechanism through which one class of axons coordinates the temporal targeting of another class of axons.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Corticothalamic Axons Are Essential for Retinal Ganglion Cell Axon Targeting to the Mouse Dorsal Lateral Geniculate Nucleus.J Neurosci. 2016 May 11;36(19):5252-63. doi: 10.1523/JNEUROSCI.4599-15.2016. J Neurosci. 2016. PMID: 27170123 Free PMC article.
-
Retinal input regulates the timing of corticogeniculate innervation.J Neurosci. 2013 Jun 12;33(24):10085-97. doi: 10.1523/JNEUROSCI.5271-12.2013. J Neurosci. 2013. PMID: 23761904 Free PMC article.
-
Reciprocal Connections Between Cortex and Thalamus Contribute to Retinal Axon Targeting to Dorsal Lateral Geniculate Nucleus.Cereb Cortex. 2018 Apr 1;28(4):1168-1182. doi: 10.1093/cercor/bhx028. Cereb Cortex. 2018. PMID: 28334242 Free PMC article.
-
How much feedback from visual cortex to lateral geniculate nucleus in cat: a perspective.Vis Neurosci. 2004 Jul-Aug;21(4):487-500. doi: 10.1017/S0952523804214018. Vis Neurosci. 2004. PMID: 15579216 Review.
-
Organization of the dorsal lateral geniculate nucleus in the mouse.Vis Neurosci. 2017 Jan;34:E008. doi: 10.1017/S0952523817000062. Vis Neurosci. 2017. PMID: 28965501 Free PMC article. Review.
Cited by
-
Not a one-trick pony: Diverse connectivity and functions of the rodent lateral geniculate complex.Vis Neurosci. 2017 Jan;34:E012. doi: 10.1017/S0952523817000098. Vis Neurosci. 2017. PMID: 28965517 Free PMC article. Review.
-
Corticothalamic Axons Are Essential for Retinal Ganglion Cell Axon Targeting to the Mouse Dorsal Lateral Geniculate Nucleus.J Neurosci. 2016 May 11;36(19):5252-63. doi: 10.1523/JNEUROSCI.4599-15.2016. J Neurosci. 2016. PMID: 27170123 Free PMC article.
-
Retinal Input Is Required for the Maintenance of Neuronal Laminae in the Ventrolateral Geniculate Nucleus.eNeuro. 2024 Sep 3;11(9):ENEURO.0022-24.2024. doi: 10.1523/ENEURO.0022-24.2024. Print 2024 Sep. eNeuro. 2024. PMID: 39160068 Free PMC article.
-
Building thalamic neuronal networks during mouse development.Front Neural Circuits. 2023 Feb 3;17:1098913. doi: 10.3389/fncir.2023.1098913. eCollection 2023. Front Neural Circuits. 2023. PMID: 36817644 Free PMC article. Review.
-
Inflammatory demyelination alters subcortical visual circuits.J Neuroinflammation. 2017 Aug 18;14(1):162. doi: 10.1186/s12974-017-0936-0. J Neuroinflammation. 2017. PMID: 28821276 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

