In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis
- PMID: 24178679
- PMCID: PMC8734557
- DOI: 10.1007/s00429-013-0660-1
In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis
Abstract
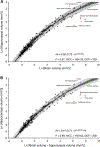
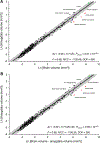
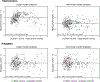
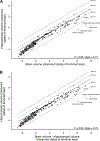
The hippocampus is essential for the formation and retrieval of memories and is a crucial neural structure sub-serving complex cognition. Adult hippocampal neurogenesis, the birth, migration and integration of new neurons, is thought to contribute to hippocampal circuit plasticity to augment function. We evaluated hippocampal volume in relation to brain volume in 375 mammal species and examined 71 mammal species for the presence of adult hippocampal neurogenesis using immunohistochemistry for doublecortin, an endogenous marker of immature neurons that can be used as a proxy marker for the presence of adult neurogenesis. We identified that the hippocampus in cetaceans (whales, dolphins and porpoises) is both absolutely and relatively small for their overall brain size, and found that the mammalian hippocampus scaled as an exponential function in relation to brain volume. In contrast, the amygdala was found to scale as a linear function of brain volume, but again, the relative size of the amygdala in cetaceans was small. The cetacean hippocampus lacks staining for doublecortin in the dentate gyrus and thus shows no clear signs of adult hippocampal neurogenesis. This lack of evidence of adult hippocampal neurogenesis, along with the small hippocampus, questions current assumptions regarding cognitive abilities associated with hippocampal function in the cetaceans. These anatomical features of the cetacean hippocampus may be related to the lack of postnatal sleep, causing a postnatal cessation of hippocampal neurogenesis.
Figures



Similar articles
-
Microbats appear to have adult hippocampal neurogenesis, but post-capture stress causes a rapid decline in the number of neurons expressing doublecortin.Neuroscience. 2014 Sep 26;277:724-33. doi: 10.1016/j.neuroscience.2014.07.063. Epub 2014 Aug 7. Neuroscience. 2014. PMID: 25106130
-
Electroconvulsive Shock, but Not Transcranial Magnetic Stimulation, Transiently Elevates Cell Proliferation in the Adult Mouse Hippocampus.Cells. 2021 Aug 14;10(8):2090. doi: 10.3390/cells10082090. Cells. 2021. PMID: 34440859 Free PMC article.
-
Developmental and adult GAP-43 deficiency in mice dynamically alters hippocampal neurogenesis and mossy fiber volume.Dev Neurosci. 2014;36(1):44-63. doi: 10.1159/000357840. Epub 2014 Feb 26. Dev Neurosci. 2014. PMID: 24576816 Free PMC article.
-
Adult neurogenesis in the mammalian dentate gyrus.Anat Histol Embryol. 2020 Jan;49(1):3-16. doi: 10.1111/ahe.12496. Epub 2019 Sep 30. Anat Histol Embryol. 2020. PMID: 31568602 Review.
-
Neurogenesis and hippocampal plasticity in adult brain.Curr Top Behav Neurosci. 2013;15:31-48. doi: 10.1007/7854_2012_217. Curr Top Behav Neurosci. 2013. PMID: 22879073 Review.
Cited by
-
GSK-3β activation accelerates early-stage consumption of Hippocampal Neurogenesis in senescent mice.Theranostics. 2020 Aug 1;10(21):9674-9685. doi: 10.7150/thno.43829. eCollection 2020. Theranostics. 2020. PMID: 32863953 Free PMC article.
-
Remembering rewarding futures: A simulation-selection model of the hippocampus.Hippocampus. 2018 Dec;28(12):913-930. doi: 10.1002/hipo.23023. Epub 2018 Nov 6. Hippocampus. 2018. PMID: 30155938 Free PMC article.
-
Organization of the sleep-related neural systems in the brain of the minke whale (Balaenoptera acutorostrata).J Comp Neurol. 2016 Jul 1;524(10):2018-35. doi: 10.1002/cne.23931. Epub 2015 Nov 30. J Comp Neurol. 2016. PMID: 26588800 Free PMC article.
-
Forebrain neuroanatomy of the neonatal and juvenile dolphin (T. truncatus and S. coeruloalba).Front Neuroanat. 2015 Nov 6;9:140. doi: 10.3389/fnana.2015.00140. eCollection 2015. Front Neuroanat. 2015. PMID: 26594155 Free PMC article.
-
Adult Neurogenesis 50 Years Later: Limits and Opportunities in Mammals.Front Neurosci. 2016 Feb 19;10:44. doi: 10.3389/fnins.2016.00044. eCollection 2016. Front Neurosci. 2016. PMID: 26924960 Free PMC article. No abstract available.
References
-
- Alme CB, Buzzetti RA, Marrone DF, Leutgeb JK, Chawla MK, Schaner MJ, Bohanik JD, Khoboko T, Leutgeb S, Moser EI, Moser MB, McNaughton BL, Barnes CA (2010) Hippocampal granule cells opt for early retirement. Hippocampus 20:1109–1123 - PubMed
-
- Alpár A, Kunzle H, Gartner U, Popkova Y, Bauer U, Grosche J, Reichenbach A, Hartig W (2010) Slow age-dependent decline of doublecortin expression and BrdU labeling in the forebrain from lesser hedgehog tenrecs. Brain Res 1330:9–19 - PubMed
-
- Amrein I, Slomianka L (2010) A morphologically distinct granule cell type in the dentate gyrus of the red fox correlates with adult hippocampal neurogenesis. Brain Res 1328:12–24 - PubMed
-
- Amrein I, Slomianka L, Poletaeva II, Bologova NV, Lipp HP (2004) Marked species and age-dependent differences in cell proliferation and neurogenesis in the hippocampus of wild-livingrodents. Hippocampus 14:1000–1010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

