Alpha 1-Antichymotrypsin, an Inflammatory Protein Overexpressed in the Brains of Patients with Alzheimer's Disease, Induces Tau Hyperphosphorylation through c-Jun N-Terminal Kinase Activation
- PMID: 24175110
- PMCID: PMC3794555
- DOI: 10.1155/2013/606083
Alpha 1-Antichymotrypsin, an Inflammatory Protein Overexpressed in the Brains of Patients with Alzheimer's Disease, Induces Tau Hyperphosphorylation through c-Jun N-Terminal Kinase Activation
Abstract
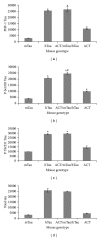
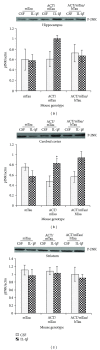
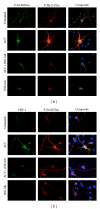
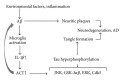
The association of inflammatory proteins with neuritic plaques in the brains of Alzheimer's disease (AD) patients has led to the hypothesis that inflammation plays a pivotal role in the development of pathology in AD. Earlier studies have shown that alpha 1-antichymotrypsin (ACT) enhances amyloid beta fibrillization and accelerated plaque formation in APP transgenic mice. Later studies from our laboratory have shown that purified ACT induces tau hyperphosphorylation and degeneration in neurons. In order to understand the mechanisms by which inflammatory proteins enhance tau hyperphosphorylation, we injected interleukin-1 β (IL-1 β ) intracerebroventricularly into mice expressing human ACT, human tau, or both transgenes. It was found that the hyperphosphorylation of tau in ACT and ACT/htau mice after IL-1 β injection correlated with increased phosphorylation of c-Jun N-terminal kinase (JNK). We verified the involvement of JNK in ACT-induced tau phosphorylation by utilizing JNK inhibitors in cultured primary neurons treated with ACT, and we found that the inhibitor showed complete prevention of ACT-induced tau phosphorylation. These results indicate that JNK is one of the major kinases involved in the ACT-mediated tau hyperphosphorylation and suggest that inhibitors of this kinase may protect against inflammation-induced tau hyperphosphorylation and neurodegeneration associated with AD.
Figures

Similar articles
-
Alpha1-antichymotrypsin, an inflammatory protein overexpressed in Alzheimer's disease brain, induces tau phosphorylation in neurons.Brain. 2006 Nov;129(Pt 11):3020-34. doi: 10.1093/brain/awl255. Epub 2006 Sep 20. Brain. 2006. PMID: 16987932
-
Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies.Curr Alzheimer Res. 2005 Jan;2(1):3-18. doi: 10.2174/1567205052772713. Curr Alzheimer Res. 2005. PMID: 15977985 Review.
-
Expression of stress-activated kinases c-Jun N-terminal kinase (SAPK/JNK-P) and p38 kinase (p38-P), and tau hyperphosphorylation in neurites surrounding betaA plaques in APP Tg2576 mice.Neuropathol Appl Neurobiol. 2004 Oct;30(5):491-502. doi: 10.1111/j.1365-2990.2004.00569.x. Neuropathol Appl Neurobiol. 2004. PMID: 15488025
-
Isorhynchophylline ameliorates cognitive impairment via modulating amyloid pathology, tau hyperphosphorylation and neuroinflammation: Studies in a transgenic mouse model of Alzheimer's disease.Brain Behav Immun. 2019 Nov;82:264-278. doi: 10.1016/j.bbi.2019.08.194. Epub 2019 Aug 30. Brain Behav Immun. 2019. PMID: 31476414
-
Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice.Exp Gerontol. 2000 Sep;35(6-7):831-41. doi: 10.1016/s0531-5565(00)00149-2. Exp Gerontol. 2000. PMID: 11053674 Review.
Cited by
-
Exosomal Aβ-Binding Proteins Identified by "In Silico" Analysis Represent Putative Blood-Derived Biomarker Candidates for Alzheimer´s Disease.Int J Mol Sci. 2021 Apr 11;22(8):3933. doi: 10.3390/ijms22083933. Int J Mol Sci. 2021. PMID: 33920336 Free PMC article.
-
Alpha 1-antichymotrypsin may be a biomarker for the progression of amnestic mild cognitive impairment.Acta Neurol Belg. 2021 Apr;121(2):451-464. doi: 10.1007/s13760-019-01206-3. Epub 2019 Sep 7. Acta Neurol Belg. 2021. PMID: 31494860
-
A proteomic approach of biomarker candidate discovery for alcoholic liver cirrhosis.J Circ Biomark. 2018 Jul 16;7:1849454418788417. doi: 10.1177/1849454418788417. eCollection 2018 Jan-Dec. J Circ Biomark. 2018. PMID: 30034555 Free PMC article.
-
Increased inflammation in BA21 brain tissue from African Americans with Alzheimer's disease.Metab Brain Dis. 2020 Jan;35(1):121-133. doi: 10.1007/s11011-019-00512-2. Epub 2019 Dec 10. Metab Brain Dis. 2020. PMID: 31823110
-
Multi-OMIC analysis of Huntington disease reveals a neuroprotective astrocyte state.bioRxiv [Preprint]. 2023 Sep 12:2023.09.08.556867. doi: 10.1101/2023.09.08.556867. bioRxiv. 2023. Update in: Nat Commun. 2024 Aug 8;15(1):6742. doi: 10.1038/s41467-024-50626-0. PMID: 37745577 Free PMC article. Updated. Preprint.
References
-
- Silverman GA, Bird PI, Carrell RW, et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. Journal of Biological Chemistry. 2001;276(36):33293–33296. - PubMed
-
- Kalsheker NA. α1-antichymotrypsin. International Journal of Biochemistry and Cell Biology. 1996;28(9):961–964. - PubMed
-
- Licastro F, Mallory M, Hansen LA, Masliah E. Increased levels of α-1-antichymotrypsin in brains of patients with Alzheimer’s disease correlate with activated astrocytes and are affected by APOE 4 genotype. Journal of Neuroimmunology. 1998;88(1-2):105–110. - PubMed
-
- Abraham CR, Selkoe DJ, Potter H. Immunochemical identification of the serine protease inhibitor α1-antichymotrypsin in the brain amyloid deposits of Alzheimer’s disease. Cell. 1988;52(4):487–501. - PubMed
-
- Licastro F, Parnetti L, Morini MC, et al. Acute phase reactant α1-antichymotrypsin is increased in cerebrospinal fluid and serum of patients with probable Alzheimer disease. Alzheimer Disease and Associated Disorders. 1995;9(2):112–118. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

