Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids
- PMID: 24161076
- PMCID: PMC4030715
- DOI: 10.1146/annurev-physiol-021113-170341
Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids
Abstract
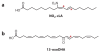
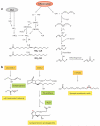
Unsaturated fatty acids are metabolized to reactive products that can act as pro- or anti-inflammatory signaling mediators. Electrophilic fatty acid species, including nitro- and oxo-containing fatty acids, display salutary anti-inflammatory and metabolic actions. Electrophilicity can be conferred by both enzymatic and oxidative reactions, via the homolytic addition of nitrogen dioxide to a double bond or via the formation of α,β-unsaturated carbonyl and epoxide substituents. The endogenous formation of electrophilic fatty acids is significant and influenced by diet, metabolic, and inflammatory reactions. Transcriptional regulatory proteins and enzymes can sense the redox status of the surrounding environment upon electrophilic fatty acid adduction of functionally significant, nucleophilic cysteines. Through this covalent and often reversible posttranslational modification, gene expression and metabolic responses are induced. At low concentrations, the pleiotropic signaling actions that are regulated by these protein targets suggest that some classes of electrophilic lipids may be useful for treating metabolic and inflammatory diseases.
Figures
Similar articles
-
Primary saturation of α, β-unsaturated carbonyl containing fatty acids does not abolish electrophilicity.Chem Biol Interact. 2021 Dec 1;350:109689. doi: 10.1016/j.cbi.2021.109689. Epub 2021 Oct 8. Chem Biol Interact. 2021. PMID: 34634267 Free PMC article.
-
Nitro-fatty acids: formation, redox signaling, and therapeutic potential.Antioxid Redox Signal. 2013 Oct 10;19(11):1257-65. doi: 10.1089/ars.2012.5023. Epub 2012 Dec 20. Antioxid Redox Signal. 2013. PMID: 23256873 Review.
-
Olives and olive oil are sources of electrophilic fatty acid nitroalkenes.PLoS One. 2014 Jan 14;9(1):e84884. doi: 10.1371/journal.pone.0084884. eCollection 2014. PLoS One. 2014. PMID: 24454759 Free PMC article.
-
Generation and esterification of electrophilic fatty acid nitroalkenes in triacylglycerides.Free Radic Biol Med. 2015 Oct;87:113-24. doi: 10.1016/j.freeradbiomed.2015.05.033. Epub 2015 Jun 9. Free Radic Biol Med. 2015. PMID: 26066303 Free PMC article.
-
Anti-inflammatory signaling actions of electrophilic nitro-arachidonic acid in vascular cells and astrocytes.Arch Biochem Biophys. 2017 Mar 1;617:155-161. doi: 10.1016/j.abb.2016.10.003. Epub 2016 Oct 5. Arch Biochem Biophys. 2017. PMID: 27720684 Review.
Cited by
-
Metabolomic profiling of samples from pediatric patients with asthma unveils deficient nutrients in African Americans.iScience. 2022 Jun 22;25(7):104650. doi: 10.1016/j.isci.2022.104650. eCollection 2022 Jul 15. iScience. 2022. PMID: 35811841 Free PMC article.
-
Short-chain fatty acids as modulators of redox signaling in health and disease.Redox Biol. 2021 Nov;47:102165. doi: 10.1016/j.redox.2021.102165. Epub 2021 Oct 14. Redox Biol. 2021. PMID: 34662811 Free PMC article. Review.
-
The Chemical Basis of Thiol Addition to Nitro-conjugated Linoleic Acid, a Protective Cell-signaling Lipid.J Biol Chem. 2017 Jan 27;292(4):1145-1159. doi: 10.1074/jbc.M116.756288. Epub 2016 Dec 6. J Biol Chem. 2017. PMID: 27923813 Free PMC article.
-
Omega-3 and -6 fatty acids allocate somatic and germline lipids to ensure fitness during nutrient and oxidative stress in Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2015 Dec 15;112(50):15378-83. doi: 10.1073/pnas.1514012112. Epub 2015 Nov 30. Proc Natl Acad Sci U S A. 2015. PMID: 26621724 Free PMC article.
-
Inflammation and Oxidative Stress Induced by Obesity, Gestational Diabetes, and Preeclampsia in Pregnancy: Role of High-Density Lipoproteins as Vectors for Bioactive Compounds.Antioxidants (Basel). 2023 Oct 23;12(10):1894. doi: 10.3390/antiox12101894. Antioxidants (Basel). 2023. PMID: 37891973 Free PMC article. Review.
References
LITERATURE CITED
-
- Singh J, Petter RC, Baillie TA, Whitty A. The resurgence of covalent drugs. Nat. Rev. Drug Discov. 2011;10:307–17. - PubMed
-
- Bottcher C, Pollmann S. Plant oxylipins: plant responses to 12-oxo-phytodienoic acid are governed by its specific structural and functional properties. FEBS J. 2009;276:4693–704. - PubMed
-
- Gersch M, Kreuzer J, Sieber SA. Electrophilic natural products and their biological targets. Nat. Prod. Rep. 2012;29:659–82. - PubMed
-
- Singer P, Shapiro H, Theilla M, Anbar R, Singer J, Cohen J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: novel mechanisms and an integrative perspective. Intensiv. Care Med. 2008;34:1580–92. - PubMed
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

