The effects of inflammatory cytokines on lymphatic endothelial barrier function
- PMID: 24141404
- PMCID: PMC4314095
- DOI: 10.1007/s10456-013-9393-2
The effects of inflammatory cytokines on lymphatic endothelial barrier function
Abstract
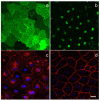
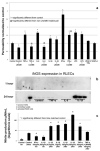
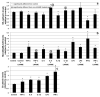
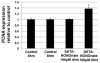
Proper lymphatic function is necessary for the transport of fluids, macromolecules, antigens and immune cells out of the interstitium. The lymphatic endothelium plays important roles in the modulation of lymphatic contractile activity and lymph transport, but it's role as a barrier between the lymph and interstitial compartments is less well understood. Alterations in lymphatic function have long been associated with edema and inflammation although the integrity of the lymphatic endothelial barrier during inflammation is not well-defined. In this paper we evaluated the integrity of the lymphatic barrier in response to inflammatory stimuli commonly associated with increased blood endothelial permeability. We utilized in vitro assays of lymphatic endothelial cell (LEC) monolayer barrier function after treatment with different inflammatory cytokines and signaling molecules including TNF-α, IL-6, IL-1β, IFN-γ and LPS. Moderate increases in an index of monolayer barrier dysfunction were noted with all treatments (20-60 % increase) except IFN-γ which caused a greater than 2.5-fold increase. Cytokine-induced barrier dysfunction was blocked or reduced by the addition of LNAME, except for IL-1β and LPS treatments, suggesting a regulatory role for nitric oxide. The decreased LEC barrier was associated with modulation of both intercellular adhesion and intracellular cytoskeletal activation. Cytokine treatments reduced the expression of VE-cadherin and increased scavenging of β-catenin in the LECs and this was partially reversed by LNAME. Likewise the phosphorylation of myosin light chain 20 at the regulatory serine 19 site, which accompanied the elevated monolayer barrier dysfunction in response to cytokine treatment, was also blunted by LNAME application. This suggests that the lymphatic barrier is regulated during inflammation and that certain inflammatory signals may induce large increases in permeability.
Figures

Similar articles
-
Differential cytokine responses in human and mouse lymphatic endothelial cells to cytokines in vitro.Lymphat Res Biol. 2010 Sep;8(3):155-64. doi: 10.1089/lrb.2010.0004. Lymphat Res Biol. 2010. PMID: 20863268 Free PMC article.
-
Ethanol-induced lymphatic endothelial cell permeability via MAP-kinase regulation.Am J Physiol Cell Physiol. 2021 Jul 1;321(1):C104-C116. doi: 10.1152/ajpcell.00039.2021. Epub 2021 Apr 28. Am J Physiol Cell Physiol. 2021. PMID: 33909502 Free PMC article.
-
Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers.J Biol Chem. 2010 Mar 5;285(10):7045-55. doi: 10.1074/jbc.M109.079277. Epub 2010 Jan 4. J Biol Chem. 2010. PMID: 20048167 Free PMC article.
-
Dynamic Regulation of Vascular Permeability by Vascular Endothelial Cadherin-Mediated Endothelial Cell-Cell Junctions.J Nippon Med Sch. 2017;84(4):148-159. doi: 10.1272/jnms.84.148. J Nippon Med Sch. 2017. PMID: 28978894 Review.
-
Rap1 Small GTPase Regulates Vascular Endothelial-Cadherin-Mediated Endothelial Cell-Cell Junctions and Vascular Permeability.Biol Pharm Bull. 2021;44(10):1371-1379. doi: 10.1248/bpb.b21-00504. Biol Pharm Bull. 2021. PMID: 34602545 Review.
Cited by
-
Emerging Roles of Mast Cells in the Regulation of Lymphatic Immuno-Physiology.Front Immunol. 2020 Jun 17;11:1234. doi: 10.3389/fimmu.2020.01234. eCollection 2020. Front Immunol. 2020. PMID: 32625213 Free PMC article. Review.
-
Targeting Lymphatics for Nanoparticle Drug Delivery.Front Pharmacol. 2022 Jun 3;13:887402. doi: 10.3389/fphar.2022.887402. eCollection 2022. Front Pharmacol. 2022. PMID: 35721179 Free PMC article. Review.
-
Bacterial Lymphatic Metastasis in Infection and Immunity.Cells. 2021 Dec 23;11(1):33. doi: 10.3390/cells11010033. Cells. 2021. PMID: 35011595 Free PMC article. Review.
-
The Role of Lymphatic Vascular Function in Metabolic Disorders.Front Physiol. 2020 May 5;11:404. doi: 10.3389/fphys.2020.00404. eCollection 2020. Front Physiol. 2020. PMID: 32477160 Free PMC article. Review.
-
Lymphangion-chip: a microphysiological system which supports co-culture and bidirectional signaling of lymphatic endothelial and muscle cells.Lab Chip. 2021 Dec 21;22(1):121-135. doi: 10.1039/d1lc00720c. Lab Chip. 2021. PMID: 34850797 Free PMC article.
References
-
- Casley-Smith JR. How the lymphatic system works. Lymphology. 1968;1(3):77–80. - PubMed
-
- Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24(2):203–215. doi:10.1016/j.immuni.2006.01.003. - PubMed
-
- Angeli V, Randolph GJ. Inflammation, lymphatic function, and dendritic cell migration. Lymphat Res Biol. 2006;4(4):217–228. doi:10.1089/lrb.2006.4406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

