Chronic cellular imaging of entire cortical columns in awake mice using microprisms
- PMID: 24139817
- PMCID: PMC3840091
- DOI: 10.1016/j.neuron.2013.07.052
Chronic cellular imaging of entire cortical columns in awake mice using microprisms
Abstract
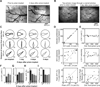
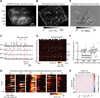
Two-photon imaging of cortical neurons in vivo has provided unique insights into the structure, function, and plasticity of cortical networks, but this method does not currently allow simultaneous imaging of neurons in the superficial and deepest cortical layers. Here, we describe a simple modification that enables simultaneous, long-term imaging of all cortical layers. Using a chronically implanted glass microprism in barrel cortex, we could image the same fluorescently labeled deep-layer pyramidal neurons across their entire somatodendritic axis for several months. We could also image visually evoked and endogenous calcium activity in hundreds of cell bodies or long-range axon terminals, across all six layers in visual cortex of awake mice. Electrophysiology and calcium imaging of evoked and endogenous activity near the prism face were consistent across days and comparable with previous observations. These experiments extend the reach of in vivo two-photon imaging to chronic, simultaneous monitoring of entire cortical columns.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Multi-layer Cortical Ca2+ Imaging in Freely Moving Mice with Prism Probes and Miniaturized Fluorescence Microscopy.J Vis Exp. 2017 Jun 13;(124):55579. doi: 10.3791/55579. J Vis Exp. 2017. PMID: 28654056 Free PMC article.
-
Integrated Microprism and Microelectrode Array for Simultaneous Electrophysiology and Two-Photon Imaging across All Cortical Layers.Adv Healthc Mater. 2024 Sep;13(24):e2302362. doi: 10.1002/adhm.202302362. Epub 2024 Apr 11. Adv Healthc Mater. 2024. PMID: 38563704
-
Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits.Nat Methods. 2020 Jan;17(1):107-113. doi: 10.1038/s41592-019-0625-2. Epub 2019 Nov 4. Nat Methods. 2020. PMID: 31686040 Free PMC article.
-
Inputs from the thalamocortical system on axon pathfinding mechanisms.Curr Opin Neurobiol. 2014 Aug;27:143-50. doi: 10.1016/j.conb.2014.03.013. Epub 2014 Apr 17. Curr Opin Neurobiol. 2014. PMID: 24742382 Review.
-
Inhibitory Circuits in Cortical Layer 5.Front Neural Circuits. 2016 May 6;10:35. doi: 10.3389/fncir.2016.00035. eCollection 2016. Front Neural Circuits. 2016. PMID: 27199675 Free PMC article. Review.
Cited by
-
Technologies for imaging neural activity in large volumes.Nat Neurosci. 2016 Aug 26;19(9):1154-64. doi: 10.1038/nn.4358. Nat Neurosci. 2016. PMID: 27571194 Free PMC article. Review.
-
Neurophotonic tools for microscopic measurements and manipulation: status report.Neurophotonics. 2022 Jan;9(Suppl 1):013001. doi: 10.1117/1.NPh.9.S1.013001. Epub 2022 Apr 27. Neurophotonics. 2022. PMID: 35493335 Free PMC article.
-
Advantages, Pitfalls, and Developments of All Optical Interrogation Strategies of Microcircuits in vivo.Front Neurosci. 2022 Jun 28;16:859803. doi: 10.3389/fnins.2022.859803. eCollection 2022. Front Neurosci. 2022. PMID: 35837124 Free PMC article. Review.
-
Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging.Nat Commun. 2014 Oct 20;5:5259. doi: 10.1038/ncomms6259. Nat Commun. 2014. PMID: 25327632 Free PMC article.
-
Imaging of Leukocyte Trafficking in Alzheimer's Disease.Front Immunol. 2016 Feb 15;7:33. doi: 10.3389/fimmu.2016.00033. eCollection 2016. Front Immunol. 2016. PMID: 26913031 Free PMC article. Review.
References
-
- Amir W, Carriles R, Hoover EE, Planchon TA, Durfee CG, Squier JA. Simultaneous imaging of multiple focal planes using a two-photon scanning microscope. Opt Lett. 2007;32:1731–1733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

