Paclitaxel-loaded nanoparticles of star-shaped cholic acid-core PLA-TPGS copolymer for breast cancer treatment
- PMID: 24134303
- PMCID: PMC3874754
- DOI: 10.1186/1556-276X-8-420
Paclitaxel-loaded nanoparticles of star-shaped cholic acid-core PLA-TPGS copolymer for breast cancer treatment
Abstract
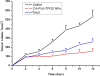
A system of novel nanoparticles of star-shaped cholic acid-core polylactide-d-α-tocopheryl polyethylene glycol 1000 succinate (CA-PLA-TPGS) block copolymer was developed for paclitaxel delivery for breast cancer treatment, which demonstrated superior in vitro and in vivo performance in comparison with paclitaxel-loaded poly(d,l-lactide-co-glycolide) (PLGA) nanoparticles and linear PLA-TPGS nanoparticles. The paclitaxel- or couramin 6-loaded nanoparticles were fabricated by a modified nanoprecipitation method and then characterized in terms of size, surface charge, surface morphology, drug encapsulation efficiency, and in vitro drug release. The CA-PLA-TPGS nanoparticles were found to be spherical in shape with an average size of around 120 nm. The nanoparticles were found to be stable, showing no change in the particle size and surface charge during 90-day storage of the aqueous solution. The release profiles of the paclitaxel-loaded nanoparticles exhibited typically biphasic release patterns. The results also showed that the CA-PLA-TPGS nanoparticles have higher antitumor efficacy than the PLA-TPGS nanoparticles and PLGA nanoparticles in vitro and in vivo. In conclusion, such nanoparticles of star-shaped cholic acid-core PLA-TPGS block copolymer could be considered as a potentially promising and effective strategy for breast cancer treatment.
Figures




Similar articles
-
Nanoparticles of star-like copolymer mannitol-functionalized poly(lactide)-vitamin E TPGS for delivery of paclitaxel to prostate cancer cells.J Biomater Appl. 2014 Sep;29(3):329-40. doi: 10.1177/0885328214527486. Epub 2014 Mar 12. J Biomater Appl. 2014. PMID: 24621530
-
Docetaxel-loaded nanoparticles based on star-shaped mannitol-core PLGA-TPGS diblock copolymer for breast cancer therapy.Acta Biomater. 2013 Nov;9(11):8910-20. doi: 10.1016/j.actbio.2013.06.034. Epub 2013 Jun 28. Acta Biomater. 2013. PMID: 23816645
-
Genistein-loaded nanoparticles of star-shaped diblock copolymer mannitol-core PLGA-TPGS for the treatment of liver cancer.Mater Sci Eng C Mater Biol Appl. 2016 Feb;59:792-800. doi: 10.1016/j.msec.2015.10.087. Epub 2015 Oct 30. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 26652434
-
Paclitaxel-loaded star-shaped copolymer nanoparticles for enhanced malignant melanoma chemotherapy against multidrug resistance.Drug Des Devel Ther. 2017 Mar 6;11:659-668. doi: 10.2147/DDDT.S127328. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28293102 Free PMC article.
-
Copolymers of poly(lactic acid) and D-α-tocopheryl polyethylene glycol 1000 succinate-based nanomedicines: versatile multifunctional platforms for cancer diagnosis and therapy.Expert Opin Drug Deliv. 2013 Apr;10(4):529-43. doi: 10.1517/17425247.2013.758632. Epub 2013 Jan 14. Expert Opin Drug Deliv. 2013. PMID: 23316695 Review.
Cited by
-
Histologic evaluation of a catheter coated with paclitaxel PLGA nanoparticles in the internal jugular veins of rats.Biomed Eng Lett. 2023 Apr 29;13(3):505-514. doi: 10.1007/s13534-023-00282-y. eCollection 2023 Aug. Biomed Eng Lett. 2023. PMID: 37519876 Free PMC article.
-
Enhanced antifungal effects of amphotericin B-TPGS-b-(PCL-ran-PGA) nanoparticles in vitro and in vivo.Int J Nanomedicine. 2014 Nov 24;9:5403-13. doi: 10.2147/IJN.S71623. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25473279 Free PMC article.
-
Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances.Pharmaceutics. 2022 Feb 4;14(2):359. doi: 10.3390/pharmaceutics14020359. Pharmaceutics. 2022. PMID: 35214091 Free PMC article. Review.
-
Improved antifungal activity of amphotericin B-loaded TPGS-b-(PCL-ran-PGA) nanoparticles.Int J Clin Exp Med. 2015 Apr 15;8(4):5150-62. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26131089 Free PMC article.
-
Release kinetics study of poorly water-soluble drugs from nanoparticles: are we doing it right?Mol Pharm. 2015 Mar 2;12(3):997-1003. doi: 10.1021/mp500817h. Epub 2015 Feb 17. Mol Pharm. 2015. PMID: 25658769 Free PMC article.
References
-
- Vivero-Escoto JL, Slowing II, Lin VS. Tuning the cellular uptake and cytotoxicity properties of oligonucleotide intercalator-functionalized mesoporous silica nanoparticles with human cervical cancer cells MCF-7. Biomaterials. 2012;8:1325–1333. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

