Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research
- PMID: 24121034
- PMCID: PMC3889862
- DOI: 10.1016/j.antiviral.2013.09.028
Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research
Abstract
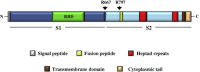
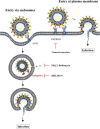
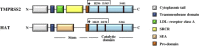
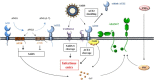
The severe acute respiratory syndrome (SARS) pandemic revealed that zoonotic transmission of animal coronaviruses (CoV) to humans poses a significant threat to public health and warrants surveillance and the development of countermeasures. The activity of host cell proteases, which cleave and activate the SARS-CoV spike (S) protein, is essential for viral infectivity and constitutes a target for intervention. However, the identities of the proteases involved have been unclear. Pioneer studies identified cathepsins and type II transmembrane serine proteases as cellular activators of SARS-CoV and demonstrated that several emerging viruses might exploit these enzymes to promote their spread. Here, we will review the proteolytic systems hijacked by SARS-CoV for S protein activation, we will discuss their contribution to viral spread in the host and we will outline antiviral strategies targeting these enzymes. This paper forms part of a series of invited articles in Antiviral Research on "From SARS to MERS: 10years of research on highly pathogenic human coronaviruses.''
Keywords: Cathepsin L; MERS; Protease; SARS; Spike protein; TMPRSS2.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein.J Virol. 2014 Jan;88(2):1293-307. doi: 10.1128/JVI.02202-13. Epub 2013 Nov 13. J Virol. 2014. PMID: 24227843 Free PMC article.
-
TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection.J Virol. 2019 Mar 5;93(6):e01815-18. doi: 10.1128/JVI.01815-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626688 Free PMC article.
-
Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2.J Virol. 2010 Dec;84(24):12658-64. doi: 10.1128/JVI.01542-10. Epub 2010 Oct 6. J Virol. 2010. PMID: 20926566 Free PMC article.
-
Differences and similarities between SARS-CoV and SARS-CoV-2: spike receptor-binding domain recognition and host cell infection with support of cellular serine proteases.Infection. 2020 Oct;48(5):665-669. doi: 10.1007/s15010-020-01486-5. Epub 2020 Jul 31. Infection. 2020. PMID: 32737833 Free PMC article. Review.
-
Receptor recognition and cross-species infections of SARS coronavirus.Antiviral Res. 2013 Oct;100(1):246-54. doi: 10.1016/j.antiviral.2013.08.014. Epub 2013 Aug 29. Antiviral Res. 2013. PMID: 23994189 Free PMC article. Review.
Cited by
-
Strengthening global health resilience: Marburg virus-like particle vaccines and the One Health approach.Sci One Health. 2024 Aug 10;3:100076. doi: 10.1016/j.soh.2024.100076. eCollection 2024. Sci One Health. 2024. PMID: 39309209 Free PMC article. Review.
-
Synergistic peptide combinations designed to suppress SARS-CoV-2.Heliyon. 2024 Apr 29;10(9):e30489. doi: 10.1016/j.heliyon.2024.e30489. eCollection 2024 May 15. Heliyon. 2024. PMID: 38726116 Free PMC article.
-
Ligand-Based Design of Selective Peptidomimetic uPA and TMPRSS2 Inhibitors with Arg Bioisosteres.Int J Mol Sci. 2024 Jan 23;25(3):1375. doi: 10.3390/ijms25031375. Int J Mol Sci. 2024. PMID: 38338655 Free PMC article.
-
SCARF Genes in COVID-19 and Kidney Disease: A Path to Comorbidity-Specific Therapies.Int J Mol Sci. 2023 Nov 8;24(22):16078. doi: 10.3390/ijms242216078. Int J Mol Sci. 2023. PMID: 38003268 Free PMC article. Review.
-
Bacterial Proteases as Potentially Exploitable Modulators of SARS-CoV-2 Infection: Logic from the Literature, Informatics, and Inspiration from the Dog.BioTech (Basel). 2023 Oct 30;12(4):61. doi: 10.3390/biotech12040061. BioTech (Basel). 2023. PMID: 37987478 Free PMC article.
References
-
- Annan A., Baldwin H.J., Corman V.M., Klose S.M., Owusu M., Nkrumah E.E., Badu E.K., Anti P., Agbenyega O., Meyer B., Oppong S., Sarkodie Y.A., Kalko E.K., Lina P.H., Godlevska E.V., Reusken C., Seebens A., Gloza-Rausch F., Vallo P., Tschapka M., Drosten C., Drexler J.F. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg. Infect. Dis. 2013;19:456–459. - PMC - PubMed
-
- Backovic M., Jardetzky T.S. Class III viral membrane fusion proteins. Adv. Exp. Med. Biol. 2011;714:91–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

