The regulatory mechanism of a client kinase controlling its own release from Hsp90 chaperone machinery through phosphorylation
- PMID: 24117238
- PMCID: PMC3927929
- DOI: 10.1042/BJ20130963
The regulatory mechanism of a client kinase controlling its own release from Hsp90 chaperone machinery through phosphorylation
Abstract
It is believed that the stability and activity of client proteins are passively regulated by the Hsp90 (heat-shock protein 90) chaperone machinery, which is known to be modulated by its intrinsic ATPase activity, co-chaperones and post-translational modifications. However, it is unclear whether client proteins themselves participate in regulation of the chaperoning process. The present study is the first example to show that a client kinase directly regulates Hsp90 activity, which is a novel level of regulation for the Hsp90 chaperone machinery. First, we prove that PKCγ (protein kinase Cγ) is a client protein of Hsp90α, and, that by interacting with PKCγ, Hsp90α prevents PKCγ degradation and facilitates its cytosol-to-membrane translocation and activation. A threonine residue set, Thr(115)/Thr(425)/Thr(603), of Hsp90α is specifically phosphorylated by PKCγ, and, more interestingly, this threonine residue set serves as a 'phosphorylation switch' for Hsp90α binding or release of PKCγ. Moreover, phosphorylation of Hsp90α by PKCγ decreases the binding affinity of Hsp90α towards ATP and co-chaperones such as Cdc37 (cell-division cycle 37), thereby decreasing its chaperone activity. Further investigation demonstrated that the reciprocal regulation of Hsp90α and PKCγ plays a critical role in cancer cells, and that simultaneous inhibition of PKCγ and Hsp90α synergistically prevents cell migration and promotes apoptosis in cancer cells.
Figures

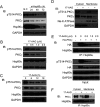


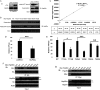
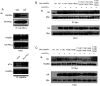
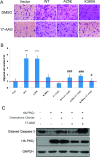

Similar articles
-
Thr90 phosphorylation of Hsp90α by protein kinase A regulates its chaperone machinery.Biochem J. 2012 Jan 1;441(1):387-97. doi: 10.1042/BJ20110855. Biochem J. 2012. PMID: 21919888
-
Chaperone Activity and Dimerization Properties of Hsp90α and Hsp90β in Glucocorticoid Receptor Activation by the Multiprotein Hsp90/Hsp70-Dependent Chaperone Machinery.Mol Pharmacol. 2018 Sep;94(3):984-991. doi: 10.1124/mol.118.112516. Epub 2018 Jun 25. Mol Pharmacol. 2018. PMID: 29941666 Free PMC article.
-
Dynamic tyrosine phosphorylation modulates cycling of the HSP90-P50(CDC37)-AHA1 chaperone machine.Mol Cell. 2012 Aug 10;47(3):434-43. doi: 10.1016/j.molcel.2012.05.015. Epub 2012 Jun 21. Mol Cell. 2012. PMID: 22727666 Free PMC article.
-
Protein kinase CK2 in health and disease: CK2: the kinase controlling the Hsp90 chaperone machinery.Cell Mol Life Sci. 2009 Jun;66(11-12):1840-9. doi: 10.1007/s00018-009-9152-0. Cell Mol Life Sci. 2009. PMID: 19387550 Free PMC article. Review.
-
Targeting the Hsp90-Cdc37-client protein interaction to disrupt Hsp90 chaperone machinery.J Hematol Oncol. 2018 Apr 27;11(1):59. doi: 10.1186/s13045-018-0602-8. J Hematol Oncol. 2018. PMID: 29699578 Free PMC article. Review.
Cited by
-
Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction.J Assist Reprod Genet. 2017 Apr;34(4):495-503. doi: 10.1007/s10815-017-0879-5. Epub 2017 Feb 24. J Assist Reprod Genet. 2017. PMID: 28236106 Free PMC article.
-
O-GlcNAcylation suppresses TRAP1 activity and promotes mitochondrial respiration.Cell Stress Chaperones. 2022 Sep;27(5):573-585. doi: 10.1007/s12192-022-01293-x. Epub 2022 Aug 17. Cell Stress Chaperones. 2022. PMID: 35976490 Free PMC article.
-
Post-translational modifications of Hsp90 and translating the chaperone code.J Biol Chem. 2020 Aug 7;295(32):11099-11117. doi: 10.1074/jbc.REV120.011833. Epub 2020 Jun 11. J Biol Chem. 2020. PMID: 32527727 Free PMC article. Review.
-
HSP90 mediates the connection of multiple programmed cell death in diseases.Cell Death Dis. 2022 Nov 5;13(11):929. doi: 10.1038/s41419-022-05373-9. Cell Death Dis. 2022. PMID: 36335088 Free PMC article. Review.
-
A novel nanodrug for the sensitization of photothermal chemotherapy for breast cancer in vitro.RSC Adv. 2024 Jul 5;14(30):21292-21299. doi: 10.1039/d4ra01611d. eCollection 2024 Jul 5. RSC Adv. 2024. PMID: 38974230 Free PMC article.
References
-
- Csermely P., Schnaider T., Soti C., Prohaszka Z., Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998;79:129–168. - PubMed
-
- Sawarkar R., Sievers C., Paro R. Hsp90 globally targets paused RNA polymerase to regulate gene expression in response to environmental stimuli. Cell. 2012;149:807–818. - PubMed
-
- Pratt W. B., Toft D. O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 2003;228:111–133. - PubMed
-
- Taipale M., Jarosz D. F., Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010;11:515–528. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

