Quantitative MRI of articular cartilage and its clinical applications
- PMID: 24115571
- PMCID: PMC3858854
- DOI: 10.1002/jmri.24313
Quantitative MRI of articular cartilage and its clinical applications
Abstract
Cartilage is one of the most essential tissues for healthy joint function and is compromised in degenerative and traumatic joint diseases. There have been tremendous advances during the past decade using quantitative MRI techniques as a noninvasive tool for evaluating cartilage, with a focus on assessing cartilage degeneration during osteoarthritis (OA). In this review, after a brief overview of cartilage composition and degeneration, we discuss techniques that grade and quantify morphologic changes as well as the techniques that quantify changes in the extracellular matrix. The basic principles, in vivo applications, advantages, and challenges for each technique are discussed. Recent studies using the OA Initiative (OAI) data are also summarized. Quantitative MRI provides noninvasive measures of cartilage degeneration at the earliest stages of joint degeneration, which is essential for efforts toward prevention and early intervention in OA.
Keywords: cartilage; osteoarthritis; quantitative MRI.
Copyright © 2013 Wiley Periodicals, Inc.
Figures



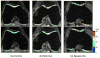
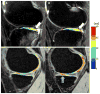
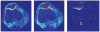
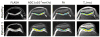
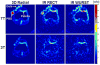
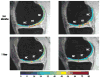
Similar articles
-
Quantitative mapping of human cartilage at 3.0T: parallel changes in T₂, T₁ρ, and dGEMRIC.Acad Radiol. 2014 Apr;21(4):463-71. doi: 10.1016/j.acra.2013.12.010. Acad Radiol. 2014. PMID: 24594416 Free PMC article.
-
Comparison of multiple quantitative MRI parameters for characterization of the goat cartilage in an ongoing osteoarthritis: dGEMRIC, T1ρ and sodium.Z Med Phys. 2016 Sep;26(3):270-82. doi: 10.1016/j.zemedi.2015.11.004. Epub 2015 Dec 24. Z Med Phys. 2016. PMID: 26725167
-
Morphologic imaging of articular cartilage.Magn Reson Imaging Clin N Am. 2011 May;19(2):229-48. doi: 10.1016/j.mric.2011.02.009. Magn Reson Imaging Clin N Am. 2011. PMID: 21665089 Review.
-
Comparison of delayed gadolinium enhanced MRI of cartilage (dGEMRIC) using inversion recovery and fast T1 mapping sequences.Magn Reson Med. 2008 Oct;60(4):768-73. doi: 10.1002/mrm.21726. Magn Reson Med. 2008. PMID: 18816842
-
T2* mapping for articular cartilage assessment: principles, current applications, and future prospects.Skeletal Radiol. 2014 Oct;43(10):1429-45. doi: 10.1007/s00256-014-1852-3. Epub 2014 Mar 19. Skeletal Radiol. 2014. PMID: 24643762 Review.
Cited by
-
Efficient phase-cycling strategy for high-resolution 3D gradient-echo quantitative parameter mapping.NMR Biomed. 2022 Jul;35(7):e4700. doi: 10.1002/nbm.4700. Epub 2022 Feb 9. NMR Biomed. 2022. PMID: 35068007 Free PMC article.
-
Patellar Dislocation in Adolescent Patients: Influence on Cartilage Properties Based on T1ρ Relaxation Times.Am J Sports Med. 2023 Dec;51(14):3714-3723. doi: 10.1177/03635465231205562. Epub 2023 Oct 28. Am J Sports Med. 2023. PMID: 37897349 Free PMC article.
-
T1ρ magnetic resonance: basic physics principles and applications in knee and intervertebral disc imaging.Quant Imaging Med Surg. 2015 Dec;5(6):858-85. doi: 10.3978/j.issn.2223-4292.2015.12.06. Quant Imaging Med Surg. 2015. PMID: 26807369 Free PMC article. Review.
-
Femoral Cartilage Ultrasound Echo Intensity Associates with Arthroscopic Cartilage Damage.Ultrasound Med Biol. 2021 Jan;47(1):43-50. doi: 10.1016/j.ultrasmedbio.2020.09.015. Epub 2020 Oct 17. Ultrasound Med Biol. 2021. PMID: 33082054 Free PMC article.
-
New advances in MRI diagnosis of degenerative osteoarthropathy of the peripheral joints.Radiol Med. 2019 Nov;124(11):1121-1127. doi: 10.1007/s11547-019-01003-1. Epub 2019 Feb 15. Radiol Med. 2019. PMID: 30771216 Review.
References
-
- Dijkgraaf LC, de Bont LG, Boering G, Liem RS. The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg. 1995;53(10):1182–1192. - PubMed
-
- Goldring M, Goldring S. Osteoarthritis. J Cell Physiol. 2007;213(3):626–634. - PubMed
-
- Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–190. - PubMed
-
- Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67(2):206–211. doi: 10.1136/ard.2006.066183. Epub 2007/05/03. ard.2006.066183 [pii] - DOI - PubMed
-
- Eckstein F, Adam C, Sittek H, Becker C, Milz S, Schulte E, et al. Non-invasive determination of cartilage thickness throughout joint surfaces using magnetic resonance imaging. J Biomech. 1997;30(3):285–289. Epub 1997/03/01. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

