Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation
- PMID: 24098509
- PMCID: PMC3789674
- DOI: 10.1371/journal.pone.0076469
Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation
Abstract
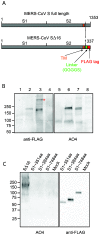
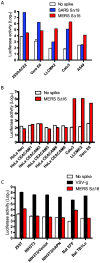
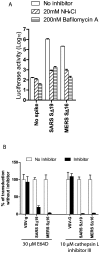
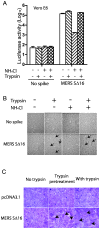
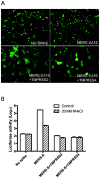
Little is known about the biology of the emerging human group c betacoronavirus, Middle East Respiratory Syndrome coronavirus (MERS-CoV). Because coronavirus spike glycoproteins (S) mediate virus entry, affect viral host range, and elicit neutralizing antibodies, analyzing the functions of MERS-CoV S protein is a high research priority. MERS-CoV S on lentivirus pseudovirions mediated entry into a variety of cell types including embryo cells from New World Eptesicus fuscus bats. Surprisingly, a polyclonal antibody to the S protein of MHV, a group a murine betacoronavirus, cross-reacted in immunoblots with the S2 domain of group c MERS-CoV spike protein. MERS pseudovirions released from 293T cells contained only uncleaved S, and pseudovirus entry was blocked by lysosomotropic reagents NH4Cl and bafilomycin and inhibitors of cathepsin L. However, when MERS pseudovirions with uncleaved S protein were adsorbed at 4°C to Vero E6 cells, brief trypsin treatment at neutral pH triggered virus entry at the plasma membrane and syncytia formation. When 293T cells producing MERS pseudotypes co-expressed serine proteases TMPRSS-2 or -4, large syncytia formed at neutral pH, and the pseudovirions produced were non-infectious and deficient in S protein. These experiments show that if S protein on MERS pseudovirions is uncleaved, then viruses enter by endocytosis in a cathepsin L-dependent manner, but if MERS-CoV S is cleaved, either during virus maturation by serine proteases or on pseudovirions by trypsin in extracellular fluids, then viruses enter at the plasma membrane at neutral pH and cause massive syncytia formation even in cells that express little or no MERS-CoV receptor. Thus, whether MERS-CoV enters cells within endosomes or at the plasma membrane depends upon the host cell type and tissue, and is determined by the location of host proteases that cleave the viral spike glycoprotein and activate membrane fusion.
Conflict of interest statement
Figures
Similar articles
-
Middle East Respiratory Syndrome Coronavirus Spike Protein Is Not Activated Directly by Cellular Furin during Viral Entry into Target Cells.J Virol. 2018 Sep 12;92(19):e00683-18. doi: 10.1128/JVI.00683-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021905 Free PMC article.
-
Coronavirus S protein-induced fusion is blocked prior to hemifusion by Abl kinase inhibitors.J Gen Virol. 2018 May;99(5):619-630. doi: 10.1099/jgv.0.001047. Epub 2018 Mar 20. J Gen Virol. 2018. PMID: 29557770 Free PMC article.
-
Ca2+ Ions Promote Fusion of Middle East Respiratory Syndrome Coronavirus with Host Cells and Increase Infectivity.J Virol. 2020 Jun 16;94(13):e00426-20. doi: 10.1128/JVI.00426-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295925 Free PMC article.
-
MERS-CoV spike protein: Targets for vaccines and therapeutics.Antiviral Res. 2016 Sep;133:165-77. doi: 10.1016/j.antiviral.2016.07.015. Epub 2016 Jul 26. Antiviral Res. 2016. PMID: 27468951 Free PMC article. Review.
-
Bat-to-human: spike features determining 'host jump' of coronaviruses SARS-CoV, MERS-CoV, and beyond.Trends Microbiol. 2015 Aug;23(8):468-78. doi: 10.1016/j.tim.2015.06.003. Epub 2015 Jul 21. Trends Microbiol. 2015. PMID: 26206723 Free PMC article. Review.
Cited by
-
Development and Evaluation of Novel Real-Time Reverse Transcription-PCR Assays with Locked Nucleic Acid Probes Targeting Leader Sequences of Human-Pathogenic Coronaviruses.J Clin Microbiol. 2015 Aug;53(8):2722-6. doi: 10.1128/JCM.01224-15. Epub 2015 May 27. J Clin Microbiol. 2015. PMID: 26019210 Free PMC article.
-
Pathophysiology of COVID-19: Mechanisms Underlying Disease Severity and Progression.Physiology (Bethesda). 2020 Sep 1;35(5):288-301. doi: 10.1152/physiol.00019.2020. Physiology (Bethesda). 2020. PMID: 32783610 Free PMC article. Review.
-
Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism.Proc Natl Acad Sci U S A. 2016 Oct 25;113(43):12262-12267. doi: 10.1073/pnas.1608147113. Epub 2016 Oct 10. Proc Natl Acad Sci U S A. 2016. PMID: 27791014 Free PMC article.
-
COVID-19/SARS-CoV-2 Infection: Lysosomes and Lysosomotropism Implicate New Treatment Strategies and Personal Risks.Int J Mol Sci. 2020 Jul 13;21(14):4953. doi: 10.3390/ijms21144953. Int J Mol Sci. 2020. PMID: 32668803 Free PMC article. Review.
-
Mutations in the Spike Protein of Middle East Respiratory Syndrome Coronavirus Transmitted in Korea Increase Resistance to Antibody-Mediated Neutralization.J Virol. 2019 Jan 4;93(2):e01381-18. doi: 10.1128/JVI.01381-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30404801 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

