Non-additive interactions between mitochondrial complex IV blockers and hypoxia in rat carotid body responses
- PMID: 24096081
- PMCID: PMC3849127
- DOI: 10.1016/j.resp.2013.09.009
Non-additive interactions between mitochondrial complex IV blockers and hypoxia in rat carotid body responses
Abstract
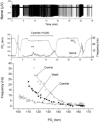
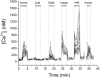
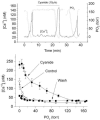
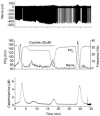
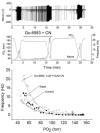
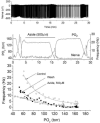
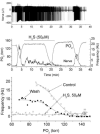
The metabolic hypothesis of carotid body chemoreceptor hypoxia transduction proposes an impairment of ATP production as the signal for activation. We hypothesized that mitochondrial complex IV blockers and hypoxia would act synergistically in exciting afferent nerve activity. Following a pre-treatment with low dosage sodium cyanide (10-20μM), the hypoxia-induced nerve response was significantly reduced along with hypoxia-induced catecholamine release. However, in isolated glomus cells, the intracellular calcium response was enhanced as initially predicted. This suggests a cyanide-mediated impairment in the step between the glomus cell intracellular calcium rise and neurotransmitter release from secretory vesicles. Administration of a PKC blocker largely reversed the inhibitory actions of cyanide on the neural response. We conclude that the expected synergism between cyanide and hypoxia occurs at the level of glomus cell intracellular calcium but not at downstream steps due to a PKC-dependent inhibition of secretion. This suggests that at least one regulatory step beyond the glomus cell calcium response may modulate the magnitude of chemoreceptor responsiveness.
Keywords: Calcium; Carotid body; Chemoreceptors; Hypoxia; Mitochondria.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Voltage- and receptor-mediated activation of a non-selective cation channel in rat carotid body glomus cells.Respir Physiol Neurobiol. 2017 Mar;237:13-21. doi: 10.1016/j.resp.2016.12.005. Epub 2016 Dec 21. Respir Physiol Neurobiol. 2017. PMID: 28013061 Free PMC article.
-
Hydrogen sulfide and hypoxia-induced changes in TASK (K2P3/9) activity and intracellular Ca(2+) concentration in rat carotid body glomus cells.Respir Physiol Neurobiol. 2015 Aug 15;215:30-8. doi: 10.1016/j.resp.2015.04.012. Epub 2015 May 5. Respir Physiol Neurobiol. 2015. PMID: 25956223 Free PMC article.
-
Influence of propofol on isolated neonatal rat carotid body glomus cell response to hypoxia and hypercapnia.Respir Physiol Neurobiol. 2019 Feb;260:17-27. doi: 10.1016/j.resp.2018.10.007. Epub 2018 Oct 30. Respir Physiol Neurobiol. 2019. PMID: 30389452 Free PMC article.
-
Autocrine and paracrine actions of ATP in rat carotid body.Can J Physiol Pharmacol. 2012 Jun;90(6):705-11. doi: 10.1139/y2012-054. Epub 2012 Apr 17. Can J Physiol Pharmacol. 2012. PMID: 22509744 Review.
-
K+ currents of glomus cells and chemosensory functions of carotid body.Respir Physiol. 1999 Apr 1;115(2):151-60. doi: 10.1016/s0034-5687(99)00021-3. Respir Physiol. 1999. PMID: 10385029 Review.
Cited by
-
Moderate inhibition of mitochondrial function augments carotid body hypoxic sensitivity.Pflugers Arch. 2016 Jan;468(1):143-155. doi: 10.1007/s00424-015-1745-x. Pflugers Arch. 2016. PMID: 26490460
-
Is Carotid Body Physiological O2 Sensitivity Determined by a Unique Mitochondrial Phenotype?Front Physiol. 2018 May 16;9:562. doi: 10.3389/fphys.2018.00562. eCollection 2018. Front Physiol. 2018. PMID: 29867584 Free PMC article. Review.
-
Hydrogen sulfide as an oxygen sensor.Antioxid Redox Signal. 2015 Feb 10;22(5):377-97. doi: 10.1089/ars.2014.5930. Epub 2014 Jul 30. Antioxid Redox Signal. 2015. PMID: 24801248 Free PMC article. Review.
References
-
- Anichkov SV, Belen'kii ML. Pharmacology of the carotid body chemoreceptors. Pergamon Press; London, England: 1963.
-
- Barnard P, Andronikou S, Pokorski M, Smatresk N, Mokashi A, Lahiri S. Time-dependent effect of hypoxia on carotid body chemosensory function. J Appl Physiol. 1987;63:685–691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

