Sirtuins in neurodegenerative diseases: an update on potential mechanisms
- PMID: 24093018
- PMCID: PMC3782645
- DOI: 10.3389/fnagi.2013.00053
Sirtuins in neurodegenerative diseases: an update on potential mechanisms
Abstract
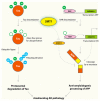
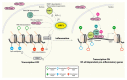
Silent information regulator 2 proteins (sirtuins or SIRTs) are a group of deacetylases (or deacylases) whose activities are dependent on and regulated by nicotinamide adenine dinucleotide (NAD(+)). Compelling evidence supports that sirtuins play major roles in many aspects of physiology, especially in pathways related to aging - the predominant and unifying risk factor for neurodegenerative diseases. In this review, we highlight the molecular mechanisms underlying the protective effects of sirtuins in neurodegenerative diseases, focusing on protein homeostasis, neural plasticity, mitochondrial function, and sustained chronic inflammation. We will also examine the potential and challenges of targeting sirtuin pathways to block these pathogenic pathways.
Keywords: NF-κB; SIRT1; amyloid-β; epigenetic regulation; inflammation; mitochondria; neurodegeneration; tau.
Figures
Similar articles
-
Protective effects and mechanisms of sirtuins in the nervous system.Prog Neurobiol. 2011 Nov;95(3):373-95. doi: 10.1016/j.pneurobio.2011.09.001. Epub 2011 Sep 10. Prog Neurobiol. 2011. PMID: 21930182 Free PMC article. Review.
-
Deciphering therapeutic options for neurodegenerative diseases: insights from SIRT1.J Mol Med (Berl). 2022 Apr;100(4):537-553. doi: 10.1007/s00109-022-02187-2. Epub 2022 Mar 11. J Mol Med (Berl). 2022. PMID: 35275221 Review.
-
Sirtuins at the Crossroads between Mitochondrial Quality Control and Neurodegenerative Diseases: Structure, Regulation, Modifications, and Modulators.Aging Dis. 2023 Jun 1;14(3):794-824. doi: 10.14336/AD.2022.1123. Aging Dis. 2023. PMID: 37191431 Free PMC article. Review.
-
Sirtuin 3 in renal diseases and aging: From mechanisms to potential therapies.Pharmacol Res. 2024 Aug;206:107261. doi: 10.1016/j.phrs.2024.107261. Epub 2024 Jun 23. Pharmacol Res. 2024. PMID: 38917912 Review.
-
Sirtuins in Neuroendocrine Regulation and Neurological Diseases.Front Neurosci. 2018 Oct 26;12:778. doi: 10.3389/fnins.2018.00778. eCollection 2018. Front Neurosci. 2018. PMID: 30416425 Free PMC article. Review.
Cited by
-
Nicotinamide riboside, an NAD+ precursor, attenuates inflammation and oxidative stress by activating sirtuin 1 in alcohol-stimulated macrophages.Lab Invest. 2021 Sep;101(9):1225-1237. doi: 10.1038/s41374-021-00599-1. Epub 2021 Apr 12. Lab Invest. 2021. PMID: 33846538
-
Sirtuin Acetylation and Deacetylation: a Complex Paradigm in Neurodegenerative Disease.Mol Neurobiol. 2021 Aug;58(8):3903-3917. doi: 10.1007/s12035-021-02387-w. Epub 2021 Apr 20. Mol Neurobiol. 2021. PMID: 33877561
-
The potential role of epigenetic modulations in BPPV maneuver exercises.Oncotarget. 2016 Jun 14;7(24):35522-35534. doi: 10.18632/oncotarget.9446. Oncotarget. 2016. PMID: 27203679 Free PMC article.
-
The Toxmatrix: Chemo-Genomic Profiling Identifies Interactions That Reveal Mechanisms of Toxicity.Chem Res Toxicol. 2018 Feb 19;31(2):127-136. doi: 10.1021/acs.chemrestox.7b00290. Epub 2017 Dec 4. Chem Res Toxicol. 2018. PMID: 29156121 Free PMC article.
-
Proteostasis in the Male and Female Germline: A New Outlook on the Maintenance of Reproductive Health.Front Cell Dev Biol. 2021 Apr 16;9:660626. doi: 10.3389/fcell.2021.660626. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937261 Free PMC article. Review.
References
-
- Albani D., Polito L., Batelli S., De Mauro S., Fracasso C., Martelli G., et al. (2009). The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1–42) peptide. J. Neurochem. 110 1445–1456 10.1111/j.1471-4159.2009.06228.x - DOI - PubMed
-
- Amat R., Planavila A., Chen S. L., Iglesias R., Giralt M., Villarroya F. (2009). SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-{gamma} co-activator-1{alpha} (PGC-1{alpha}) gene in skeletal muscle through the PGC-1{alpha} autoregulatory loop and interaction with MyoD. J. Biol. Chem. 284 21872–21880 10.1074/jbc.M109.022749 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

