On the use of L-012, a luminol-based chemiluminescent probe, for detecting superoxide and identifying inhibitors of NADPH oxidase: a reevaluation
- PMID: 24080119
- PMCID: PMC4274999
- DOI: 10.1016/j.freeradbiomed.2013.09.017
On the use of L-012, a luminol-based chemiluminescent probe, for detecting superoxide and identifying inhibitors of NADPH oxidase: a reevaluation
Abstract
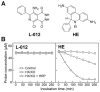
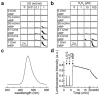
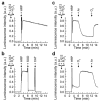
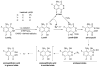
L-012, a luminol-based chemiluminescent (CL) probe, is widely used in vitro and in vivo to detect NADPH oxidase (Nox)-derived superoxide (O2(*-)) and identify Nox inhibitors. Yet understanding of the free radical chemistry of the L-012 probe is still lacking. We report that peroxidase and H2O2 induce superoxide dismutase (SOD)-sensitive, L-012-derived CL in the presence of oxygen. O2(*-) alone does not react with L-012 to emit luminescence. Self-generated O2(*-) during oxidation of L-012 and luminol analogs artifactually induce CL inhibitable by SOD. These aspects make assays based on luminol analogs less than ideal for specific detection and identification of O2(*-) and NOX inhibitors.
Keywords: 8-amino-5-chloro-7-phenylpyrido[3,4–d]pyridazine-1,4(2H,3H)dione; CAT; CL; Free radicals; HE; HRP; HX; L-012; Luminescence; Luminol; NADPH oxidase; NADPH oxidases; Nox; O(2)(−); ROS; Redox cycling; Redox probe; SOD; Superoxide radical anion; XO; catalase; chemiluminescence; diethylenetriaminepentaacetate; dtpa; horseradish peroxidase; hydroethidine; hypoxanthine; reactive oxygen species; superoxide dismutase; superoxide radical anion; xanthine oxidase.
© 2013 Published by Elsevier Inc.
Figures

Similar articles
-
Detection of superoxide and peroxynitrite in model systems and mitochondria by the luminol analogue L-012.Free Radic Res. 2004 Mar;38(3):259-69. doi: 10.1080/10715760410001659773. Free Radic Res. 2004. PMID: 15129734
-
Evaluation of lophine derivatives as L-012 (luminol analog)-dependent chemiluminescence enhancers for measuring horseradish peroxidase and H2O2.Luminescence. 2014 Mar;29(2):118-21. doi: 10.1002/bio.2513. Epub 2013 Apr 30. Luminescence. 2014. PMID: 23630098
-
Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases.Biochim Biophys Acta. 2014 Feb;1840(2):757-67. doi: 10.1016/j.bbagen.2013.04.040. Epub 2013 May 7. Biochim Biophys Acta. 2014. PMID: 23660153 Free PMC article. Review.
-
A comparison of chemical systems for luminometric determination of antioxidant capacity towards individual reactive oxygen species.Luminescence. 2006 Jul-Aug;21(4):239-44. doi: 10.1002/bio.913. Luminescence. 2006. PMID: 16791875
-
Recent developments in detection of superoxide radical anion and hydrogen peroxide: Opportunities, challenges, and implications in redox signaling.Arch Biochem Biophys. 2017 Mar 1;617:38-47. doi: 10.1016/j.abb.2016.08.021. Epub 2016 Aug 30. Arch Biochem Biophys. 2017. PMID: 27590268 Free PMC article. Review.
Cited by
-
Bortezomib is an effective enhancer for chemical probe-dependent superoxide detection.Front Med (Lausanne). 2022 Dec 21;9:941180. doi: 10.3389/fmed.2022.941180. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36619644 Free PMC article.
-
GLP-1 Analog Liraglutide Improves Vascular Function in Polymicrobial Sepsis by Reduction of Oxidative Stress and Inflammation.Antioxidants (Basel). 2021 Jul 23;10(8):1175. doi: 10.3390/antiox10081175. Antioxidants (Basel). 2021. PMID: 34439423 Free PMC article.
-
Aging Intensifies Myeloperoxidase Activity after Ischemic Stroke.Aging Dis. 2024 Aug 30;15(6):2650-2664. doi: 10.14336/AD.2023.1640. Aging Dis. 2024. PMID: 39325942 Free PMC article.
-
Wound healing after excision of subcutaneous tumors treated with near-infrared photoimmunotherapy.Cancer Med. 2020 Aug;9(16):5932-5939. doi: 10.1002/cam4.3247. Epub 2020 Jun 24. Cancer Med. 2020. PMID: 32579795 Free PMC article.
-
Small-molecule luminescent probes for the detection of cellular oxidizing and nitrating species.Free Radic Biol Med. 2018 Nov 20;128:3-22. doi: 10.1016/j.freeradbiomed.2018.03.032. Epub 2018 Mar 19. Free Radic Biol Med. 2018. PMID: 29567392 Free PMC article. Review.
References
-
- Nishinaka Y, Aramaki Y, Yoshida H, Masuya H, Sugawara T, Ichimori Y. A new sensitive chemiluminescence probe, L-012, for measuring the production of superoxide anion by cells. Biochem Biophys Res Commun. 1993;193:554–549. - PubMed
-
- Sohn HY, Gloe T, Keller M, Schoenafinger K, Pohl U. Sensitive superoxide detection in vascular cells by the new chemiluminescence dye L-012. J Vasc Res. 1999;36:456–464. - PubMed
-
- Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free Radic Biol Med. 2006;41:193–201. - PubMed
-
- Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–H32. - PubMed
-
- Daiber A, Oelze M, August M, Wendt M, Sydow K, Wieboldt H, Kleschyov AL, Munzel T. Detection of superoxide and peroxynitrite in model systems and mitochondria by the luminol analogue L-012. Free Radic Res. 2004;38:259–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

