Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide
- PMID: 24077178
- PMCID: PMC4076143
- DOI: 10.1038/nchembio.1352
Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide
Abstract
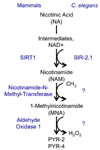
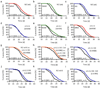
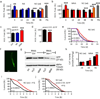
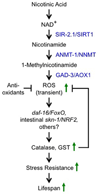
Sirtuins, a family of histone deacetylases, have a fiercely debated role in regulating lifespan. In contrast with recent observations, here we find that overexpression of sir-2.1, the ortholog of mammalian SirT1, does extend Caenorhabditis elegans lifespan. Sirtuins mandatorily convert NAD(+) into nicotinamide (NAM). We here find that NAM and its metabolite, 1-methylnicotinamide (MNA), extend C. elegans lifespan, even in the absence of sir-2.1. We identify a previously unknown C. elegans nicotinamide-N-methyltransferase, encoded by a gene now named anmt-1, to generate MNA from NAM. Disruption and overexpression of anmt-1 have opposing effects on lifespan independent of sirtuins, with loss of anmt-1 fully inhibiting sir-2.1-mediated lifespan extension. MNA serves as a substrate for a newly identified aldehyde oxidase, GAD-3, to generate hydrogen peroxide, which acts as a mitohormetic reactive oxygen species signal to promote C. elegans longevity. Taken together, sirtuin-mediated lifespan extension depends on methylation of NAM, providing an unexpected mechanistic role for sirtuins beyond histone deacetylation.
Conflict of interest statement
Figures


Comment in
-
Sirtuins: Longevity focuses on NAD+.Nat Chem Biol. 2013 Nov;9(11):666-7. doi: 10.1038/nchembio.1369. Nat Chem Biol. 2013. PMID: 24141218 No abstract available.
Similar articles
-
Pheromone sensing regulates Caenorhabditis elegans lifespan and stress resistance via the deacetylase SIR-2.1.Proc Natl Acad Sci U S A. 2013 Apr 2;110(14):5522-7. doi: 10.1073/pnas.1214467110. Epub 2013 Mar 18. Proc Natl Acad Sci U S A. 2013. PMID: 23509272 Free PMC article.
-
Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila.Nature. 2011 Sep 21;477(7365):482-5. doi: 10.1038/nature10296. Nature. 2011. PMID: 21938067 Free PMC article.
-
Sesamin extends lifespan through pathways related to dietary restriction in Caenorhabditis elegans.Eur J Nutr. 2018 Apr;57(3):1137-1146. doi: 10.1007/s00394-017-1396-0. Epub 2017 Feb 26. Eur J Nutr. 2018. PMID: 28239780
-
A stress response pathway involving sirtuins, forkheads and 14-3-3 proteins.Cell Cycle. 2006 Nov;5(22):2588-91. doi: 10.4161/cc.5.22.3513. Epub 2006 Nov 15. Cell Cycle. 2006. PMID: 17172829 Review.
-
Sirtuins: the 'magnificent seven', function, metabolism and longevity.Ann Med. 2007;39(5):335-45. doi: 10.1080/07853890701408194. Ann Med. 2007. PMID: 17701476 Review.
Cited by
-
Deep Proteome Analysis Identifies Age-Related Processes in C. elegans.Cell Syst. 2016 Aug;3(2):144-159. doi: 10.1016/j.cels.2016.06.011. Epub 2016 Jul 21. Cell Syst. 2016. PMID: 27453442 Free PMC article.
-
Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds.Nat Rev Mol Cell Biol. 2016 Nov;17(11):679-690. doi: 10.1038/nrm.2016.93. Epub 2016 Aug 24. Nat Rev Mol Cell Biol. 2016. PMID: 27552971 Free PMC article. Review.
-
Activation of the Mitochondrial Unfolded Protein Response: A New Therapeutic Target?Biomedicines. 2022 Jul 6;10(7):1611. doi: 10.3390/biomedicines10071611. Biomedicines. 2022. PMID: 35884915 Free PMC article. Review.
-
Kynurenine pathway, NAD+ synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan.Exp Gerontol. 2020 Apr;132:110841. doi: 10.1016/j.exger.2020.110841. Epub 2020 Jan 16. Exp Gerontol. 2020. PMID: 31954874 Free PMC article. Review.
-
ROS function in redox signaling and oxidative stress.Curr Biol. 2014 May 19;24(10):R453-62. doi: 10.1016/j.cub.2014.03.034. Curr Biol. 2014. PMID: 24845678 Free PMC article. Review.
References
References to Online Methods
-
- Segref A, Hoppe T. Analysis of ubiquitin-dependent proteolysis in Caenorhabditis elegans. Methods Mol Biol. 2012;832:531–544. - PubMed
-
- Janssen AJ, et al. Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin Chem. 2007;53:729–734. - PubMed
-
- Musfeld C, Biollaz J, Belaz N, Kesselring UW, Decosterd LA. Validation of an HPLC method for the determination of urinary and plasma levels of N1-methylnicotinamide, an endogenous marker of renal cationic transport and plasma flow. J Pharm Biomed Anal. 2001;24:391–404. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

