The impact of the receptor of hyaluronan-mediated motility (RHAMM) on human urothelial transitional cell cancer of the bladder
- PMID: 24069434
- PMCID: PMC3775893
- DOI: 10.1371/journal.pone.0075681
The impact of the receptor of hyaluronan-mediated motility (RHAMM) on human urothelial transitional cell cancer of the bladder
Abstract
Hyaluronan (HA) is a carbohydrate of the extracellular matrix with tumor promoting effects in a variety of cancers. The present study addressed the role of HA matrix for progression and prognosis of human bladder cancer by studying the expression and function of HA-related genes.
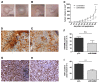
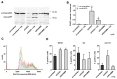
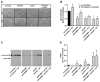
Methods: Tissue samples of 120 patients with different stages of transitional cell bladder cancer, who underwent surgical treatment for bladder cancer at the University Hospital of Essen were analysed. mRNA-expression levels of HA synthases (HAS1-3) and HA-receptors (RHAMM and CD44) were evaluated by real time RT-PCR in comparison to healthy bladder tissue as control. In uni- and multivariate cox proportional hazard survival regression analysis, the impact of the gene expression levels on survival was assessed. In vitro knock-down of RHAMM, CD44 and HAS isoenzymes was achieved by siRNA and lentiviral shRNA in J82 bladder cancer cells. Transfected cells were analysed in vitro with regard to proliferation, cell cycle and apoptosis. J82 cells after knock-down of RHAMM were xenografted into male nu/nu athymic mice to monitor tumor progression in vivo.
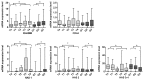
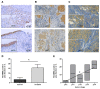
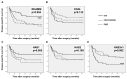
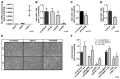
Results: In invasive tumor stages RHAMM-, HAS1 and HAS2 mRNA-expression levels were elevated whereas HAS3v1 was reduced as compared to non-invasive tumors. Subsequently, Kaplan-Meier analysis revealed reduced bladder cancer specific survival in patients with high RHAMM mRNA and low HAS3v1 expression. Elevated RHAMM in invasive tumors was confirmed by RHAMM immunohistochemistry. Furthermore, multivariate analysis revealed that only RHAMM expression was associated with poor prognosis independent from other survival factors (HR=2.389, 95% CI 1.227-4.651, p=0.01). Lentiviral RHAMM knock-down revealed reduced J82 cell proliferation in vitro and reduced xenograft tumor growth in vivo.
Conclusion: The data suggest that RHAMM plays a crucial role in mediating progression of muscle-invasive bladder cancer and recommends RHAMM for further evaluation as a prognostic marker or therapeutic target in bladder cancer therapy.
Conflict of interest statement
Figures
Similar articles
-
CD44 and RHAMM are essential for rapid growth of bladder cancer driven by loss of Glycogen Debranching Enzyme (AGL).BMC Cancer. 2016 Sep 5;16(1):713. doi: 10.1186/s12885-016-2756-5. BMC Cancer. 2016. PMID: 27595989 Free PMC article.
-
Triptolide suppresses the in vitro and in vivo growth of lung cancer cells by targeting hyaluronan-CD44/RHAMM signaling.Oncotarget. 2017 Apr 18;8(16):26927-26940. doi: 10.18632/oncotarget.15879. Oncotarget. 2017. PMID: 28460475 Free PMC article.
-
Hyaluronic acid, CD44 and RHAMM regulate myoblast behavior during embryogenesis.Matrix Biol. 2019 May;78-79:236-254. doi: 10.1016/j.matbio.2018.08.008. Epub 2018 Aug 18. Matrix Biol. 2019. PMID: 30130585 Free PMC article.
-
Role of receptor for hyaluronan-mediated motility (RHAMM) in human head and neck cancers.J Cancer Res Clin Oncol. 2014 Oct;140(10):1629-40. doi: 10.1007/s00432-014-1653-z. Epub 2014 Mar 28. J Cancer Res Clin Oncol. 2014. PMID: 24676428 Review.
-
The roles of hyaluronan/RHAMM/CD44 and their respective interactions along the insidious pathways of fibrosarcoma progression.Biomed Res Int. 2013;2013:929531. doi: 10.1155/2013/929531. Epub 2013 Sep 5. Biomed Res Int. 2013. PMID: 24083250 Free PMC article. Review.
Cited by
-
Expression of the receptor for hyaluronic acid mediated motility (RHAMM) is associated with poor prognosis and metastasis in non-small cell lung carcinoma.Oncotarget. 2016 Jun 28;7(26):39957-39969. doi: 10.18632/oncotarget.9554. Oncotarget. 2016. PMID: 27220886 Free PMC article.
-
CD44 and RHAMM are essential for rapid growth of bladder cancer driven by loss of Glycogen Debranching Enzyme (AGL).BMC Cancer. 2016 Sep 5;16(1):713. doi: 10.1186/s12885-016-2756-5. BMC Cancer. 2016. PMID: 27595989 Free PMC article.
-
Identification of the HMMR Gene as a Diagnostic and Prognostic Biomarker in Hepatocellular Carcinoma Based on Integrated Bioinformatics Analysis.Evid Based Complement Alternat Med. 2021 Jun 15;2021:5970085. doi: 10.1155/2021/5970085. eCollection 2021. Evid Based Complement Alternat Med. 2021. Retraction in: Evid Based Complement Alternat Med. 2023 Sep 27;2023:9819147. doi: 10.1155/2023/9819147. PMID: 34221079 Free PMC article. Retracted.
-
Increased RHAMM expression relates to ovarian cancer progression.J Ovarian Res. 2017 Sep 27;10(1):66. doi: 10.1186/s13048-017-0360-1. J Ovarian Res. 2017. PMID: 28954627 Free PMC article.
-
Expression patterns of CD168 correlate with the stage and grade of squamous cell carcinoma of head and neck.Mol Clin Oncol. 2017 Apr;6(4):597-602. doi: 10.3892/mco.2017.1165. Epub 2017 Feb 13. Mol Clin Oncol. 2017. PMID: 28413676 Free PMC article.
References
-
- Ploeg M, Aben KK, Kiemeney LA (2009) The present and future burden of urinary bladder cancer in the world. World J Urol 27: 289-293. doi:10.1007/s00345-009-0383-3. PubMed: 19219610. - DOI - PMC - PubMed
-
- Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, Palou J, Algaba F et al. (2000) Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol 164: 680-684. doi:10.1016/S0022-5347(05)67280-1. PubMed: 10954628. - DOI - PubMed
-
- Liberman D, Alasker A, Sun M, Ismail S, Lughezzani G et al. (2011) Radical cystectomy for patients with pT4 urothelial carcinoma in a large population-based study. BJU Int 107: 905-911. doi:10.1111/j.1464-410X.2010.09590.x. PubMed: 20860649. - DOI - PubMed
-
- Toole BP (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4: 528-539. doi:10.1038/nrc1391. PubMed: 15229478. - DOI - PubMed
-
- de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA (2003) Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-alpha-trypsin inhibitor is crucial to structure and function. Am J Pathol 163: 121-133. doi:10.1016/S0002-9440(10)63636-X. PubMed: 12819017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

