Effects of prolonged selective serotonin reuptake inhibition on the development and expression of L-DOPA-induced dyskinesia in hemi-parkinsonian rats
- PMID: 24067924
- PMCID: PMC3865178
- DOI: 10.1016/j.neuropharm.2013.09.017
Effects of prolonged selective serotonin reuptake inhibition on the development and expression of L-DOPA-induced dyskinesia in hemi-parkinsonian rats
Abstract
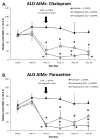
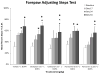
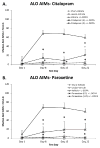
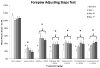
Dopamine (DA) replacement therapy with l-DOPA is the standard treatment for Parkinson's disease (PD). Unfortunately chronic treatment often leads to the development of abnormal involuntary movements (AIMs) referred to as L-DOPA-induced dyskinesia (LID). Accumulating evidence has shown that compensatory plasticity in serotonin (5-HT) neurons contributes to LID and recent work has indicated that acute 5-HT transporter (SERT) blockade provides anti-dyskinetic protection. However neither the persistence nor the mechanism(s) of these effects have been investigated. Therefore the current endeavor sought to mimic a prolonged regimen of SERT inhibition in L-DOPA-primed and -naïve hemi-parkinsonian rats. Rats received 3 weeks of daily co-treatment of the selective 5-HT reuptake inhibitors (SSRIs) citalopram (0, 3, or 5 mg/kg) or paroxetine (0, 0.5, or 1.25 mg/kg) with L-DOPA (6 mg/kg) during which AIMs and motor performance were monitored. In order to investigate potential mechanisms of action, tissue levels of striatal monoamines were monitored and the 5-HT(1A) receptor antagonist WAY100635 (0.5 mg/kg) was used. Results revealed that prolonged SSRIs attenuated AIMs expression and development in L-DOPA-primed and -naïve subjects, respectively, without interfering with motor performance. Neurochemical analysis of striatal tissue indicated that a 3 week SERT blockade increased DA levels in L-DOPA-treated rats. Pharmacologically, anti-dyskinetic effects were partially reversed with WAY100635 signifying involvement of the 5-HT1A receptor. Collectively, these findings demonstrate that prolonged SERT inhibition provides enduring anti-dyskinetic effects in part via 5-HT(1A) receptors while maintaining L-DOPA's anti-parkinsonian efficacy by enhancing striatal DA levels.
Keywords: 3,4-dihydroxyphenylacetic acid; 5-HIAA; 5-HT; 5-HT(1A) receptor; 5-hydroxyindoleacetic acid; 6-OHDA; 6-hydroxydopamine; AIMs; ALO; Abnormal involuntary movements; Axial, limb and orolingual; Benserazide; DA; DAT; DL-serine 2-(2,3,4-trihydroxybenzyl) hydrazine hydrochloride; DMSO; DOPAC; Dimethyl sulfoxide; Dopamine; Dopamine transporter; FAS; Forepaw adjusting steps test; HPLC; High performance liquid chromatography; LID; MFB; Medial forebrain bundle; N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt; NE; Norepinephrine; PD; Parkinson's disease; SERT; SSRI; Selective 5-HT reuptake inhibitor; Selective serotonin reuptake inhibitor; Serotonin; Serotonin transporter; WAY100635; l-DOPA-induced dyskinesia.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Serotonin transporter inhibition attenuates l-DOPA-induced dyskinesia without compromising l-DOPA efficacy in hemi-parkinsonian rats.Eur J Neurosci. 2012 Sep;36(6):2839-48. doi: 10.1111/j.1460-9568.2012.08202.x. Epub 2012 Jul 5. Eur J Neurosci. 2012. PMID: 22762478 Free PMC article.
-
The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy.Pharmacol Biochem Behav. 2007 Aug-Sep;87(3):306-14. doi: 10.1016/j.pbb.2007.05.002. Epub 2007 May 13. Pharmacol Biochem Behav. 2007. PMID: 17553556
-
Diverse serotonin actions of vilazodone reduce l-3,4-dihidroxyphenylalanine-induced dyskinesia in hemi-parkinsonian rats.Mov Disord. 2018 Nov;33(11):1740-1749. doi: 10.1002/mds.100. Mov Disord. 2018. PMID: 30485908
-
Parkinson's disease--opportunities for novel therapeutics to reduce the problems of levodopa therapy.Prog Brain Res. 2008;172:479-94. doi: 10.1016/S0079-6123(08)00923-0. Prog Brain Res. 2008. PMID: 18772047 Review.
-
Serotonin 5-HT1A receptors and their interactions with adenosine A2A receptors in Parkinson's disease and dyskinesia.Neuropharmacology. 2023 Mar 15;226:109411. doi: 10.1016/j.neuropharm.2023.109411. Epub 2023 Jan 4. Neuropharmacology. 2023. PMID: 36608814 Review.
Cited by
-
Monoamine reuptake inhibitors in Parkinson's disease.Parkinsons Dis. 2015;2015:609428. doi: 10.1155/2015/609428. Epub 2015 Feb 25. Parkinsons Dis. 2015. PMID: 25810948 Free PMC article. Review.
-
Role of 5-HT1A Receptor in Vilazodone-Mediated Suppression of L-DOPA-Induced Dyskinesia and Increased Responsiveness to Cortical Input in Striatal Medium Spiny Neurons in an Animal Model of Parkinson's Disease.Molecules. 2021 Sep 24;26(19):5790. doi: 10.3390/molecules26195790. Molecules. 2021. PMID: 34641332 Free PMC article.
-
Serotonergic targets for the treatment of L-DOPA-induced dyskinesia.J Neural Transm (Vienna). 2018 Aug;125(8):1203-1216. doi: 10.1007/s00702-017-1837-1. Epub 2018 Jan 5. J Neural Transm (Vienna). 2018. PMID: 29305656 Review.
-
The effect of triple reuptake inhibitor toludesvenlafaxine on neurological function in cerebral ischemic rats.Front Pharmacol. 2023 Apr 21;14:1073099. doi: 10.3389/fphar.2023.1073099. eCollection 2023. Front Pharmacol. 2023. PMID: 37153779 Free PMC article.
-
Serotonergic Regulation of Synaptic Dopamine Levels Mitigates L-DOPA-Induced Dyskinesia in a Mouse Model of Parkinson's Disease.J Parkinsons Dis. 2024;14(5):941-964. doi: 10.3233/JPD-240080. J Parkinsons Dis. 2024. PMID: 38905058 Free PMC article.
References
-
- Arai R, Karasawa N, Geffard M, Nagatsu I. L-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a double-labeling immunofluoresence study. Neurosci Lett. 1995;195:195–198. - PubMed
-
- Bara-Jiminez W, Bibbiani F, Morris M, Dimitrova T, Sherzai A, Mouradian M, Chase T. Effects of serotonin 5-HT1A agonist in advanced Parkinson’s disease. Mov Disord. 2005;20:932–936. - PubMed
-
- Bibbiani F, Oh J, Chase T. Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurol. 2001;57:1829–1834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

