Prolonged glycation of hen egg white lysozyme generates non amyloidal structures
- PMID: 24066139
- PMCID: PMC3774808
- DOI: 10.1371/journal.pone.0074336
Prolonged glycation of hen egg white lysozyme generates non amyloidal structures
Abstract
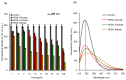
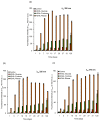
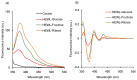
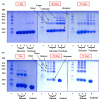
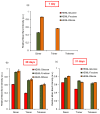
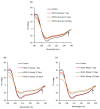
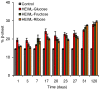
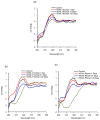
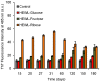
Glycation causes severe damage to protein structure that could lead to amyloid formation in special cases. Here in this report, we have shown for the first time that hen egg white lysozyme (HEWL) does not undergo amyloid formation even after prolonged glycation in the presence of D-glucose, D-fructose and D-ribose. Cross-linked oligomers were formed in all the cases and ribose was found to be the most potent among the three sugars. Ribose mediated oligomers, however, exhibit Thioflavin T binding properties although microscopic images clearly show amorphous and globular morphology of the aggregates. Our study demonstrates that the structural damage of hen egg white lysozyme due to glycation generates unstructured aggregates.
Conflict of interest statement
Figures

Similar articles
-
Mechanistic insights in glycation-induced protein aggregation.Biomacromolecules. 2014 Sep 8;15(9):3449-62. doi: 10.1021/bm501077j. Epub 2014 Aug 1. Biomacromolecules. 2014. PMID: 25057908
-
Influence of the ionic strength on the amyloid fibrillogenesis of hen egg white lysozyme.Int J Biol Macromol. 2019 Jan;121:63-70. doi: 10.1016/j.ijbiomac.2018.09.165. Epub 2018 Oct 2. Int J Biol Macromol. 2019. PMID: 30290259
-
The effect of concentration, temperature and stirring on hen egg white lysozyme amyloid formation.Soft Matter. 2013 Oct 28;9(40):9692-701. doi: 10.1039/c3sm51671g. Soft Matter. 2013. PMID: 26029778
-
Biophysical insights into the interaction of hen egg white lysozyme with therapeutic dye clofazimine: modulation of activity and SDS induced aggregation of model protein.J Biomol Struct Dyn. 2017 Aug;35(10):2197-2210. doi: 10.1080/07391102.2016.1211552. Epub 2016 Aug 5. J Biomol Struct Dyn. 2017. PMID: 27400444
-
Lysozyme: a model enzyme in protein crystallography.EXS. 1996;75:185-222. doi: 10.1007/978-3-0348-9225-4_11. EXS. 1996. PMID: 8765301 Review.
Cited by
-
Glyoxal modification mediates conformational alterations in silk fibroin: Induction of fibrillation with amyloidal features.J Biosci. 2020;45:32. J Biosci. 2020. Retraction in: J Biosci. 2020;45:142. PMID: 32098911 Retracted.
-
Understanding the Role of Protein Glycation in the Amyloid Aggregation Process.Int J Mol Sci. 2021 Jun 21;22(12):6609. doi: 10.3390/ijms22126609. Int J Mol Sci. 2021. PMID: 34205510 Free PMC article. Review.
-
Structural and Functional Characterization of Covalently Modified Proteins Formed By a Glycating Agent, Glyoxal.ACS Omega. 2021 Aug 9;6(32):20887-20894. doi: 10.1021/acsomega.1c02300. eCollection 2021 Aug 17. ACS Omega. 2021. PMID: 34423196 Free PMC article.
-
Formation of Pentosidine Cross-Linking in Myoglobin by Glyoxal: Detection of Fluorescent Advanced Glycation End Product.J Fluoresc. 2017 Jul;27(4):1213-1219. doi: 10.1007/s10895-017-2064-8. Epub 2017 Mar 15. J Fluoresc. 2017. PMID: 28299531
-
An overview on glycation: molecular mechanisms, impact on proteins, pathogenesis, and inhibition.Biophys Rev. 2024 Apr 12;16(2):189-218. doi: 10.1007/s12551-024-01188-4. eCollection 2024 Apr. Biophys Rev. 2024. PMID: 38737201 Free PMC article. Review.
References
-
- Cloos PAC, Christgau S (2002) Non-enzymatic covalent modifications of proteins: mechanisms, physiological consequences and clinical applications. Matrix Biol 21: 39–52. doi:10.1016/S0945-053X(01)00188-3. PubMed: 11827791. - DOI - PubMed
-
- Lapolla A, Traldi P, Fedele D (2005) Importance of measuring products of non-enzymatic glycation of proteins. Clin Biochem 38: 103–115. doi:10.1016/j.clinbiochem.2004.09.007. PubMed: 15642271. - DOI - PubMed
-
- Brownlee M (1995) Advanced protein glycosylation in diabetes and aging. Annu Rev Med 46: 223–234. doi:10.1146/annurev.med.46.1.223. PubMed: 7598459. - DOI - PubMed
-
- Bouma B, Kroon-Batenburg LMJ, Wu YP, Brünjes B, Posthuma G et al. (2003) Glycation induces formation of amyloid cross-β structure in albumin. J Biol Chem 278: 41810–41819. doi:10.1074/jbc.M303925200. PubMed: 12909637. - DOI - PubMed
-
- Chevalier F, Chobert JM, Dalgalarrondo M, Choiset Y, Haertlé T (2002) Maillard glycation of beta-lactoglobulin induces conformation changes. Nahrung 46: 58–63. doi:10.1002/1521-3803(20020301)46:2. PubMed: 12017991. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

